Abstract
Since the 2007 Diabetes Surgery Summit in Rome, Italy, and the subsequent publishing of the world’s first guidelines for the surgical treatment of type 2 diabetes (T2D), much new evidence regarding the efficacy and safety of metabolic surgery has emerged. Additional observational cohort studies support the superior effects of surgery over medical treatment with respect to glycemic control, weight loss, and even reduction in mortality and microvascular complications associated with T2D. Furthermore, new safety data suggest that the perioperative morbidity and mortality of metabolic surgery (5% and 0.3%, respectively) are now similar to that of common low-risk procedures, such as cholecystectomy and hysterectomy. The largest advance, however, has been the completion of 11 randomized controlled trials from around the globe that compare surgery with medical treatment of T2D. These studies with follow-up duration of 1–5 years involve nearly 800 patients without surgical mortality and with major complication rates of less than 5% and a reoperation rate of 8%. All but 1 of the 11 randomized controlled trials have shown the superiority of surgery over medical management at achieving remission or glycemic improvement. Surgery was also superior to medical treatment with respect to improving cardiovascular risk factors, such as weight loss and dyslipidemia, while reducing medication burden. This new efficacy and safety evidence should help guide physicians across the globe to the appropriate use of surgery as an effective treatment for patients suffering from T2D and obesity.
Surgical Procedures for Severe Obesity and Metabolic Disease and Indications
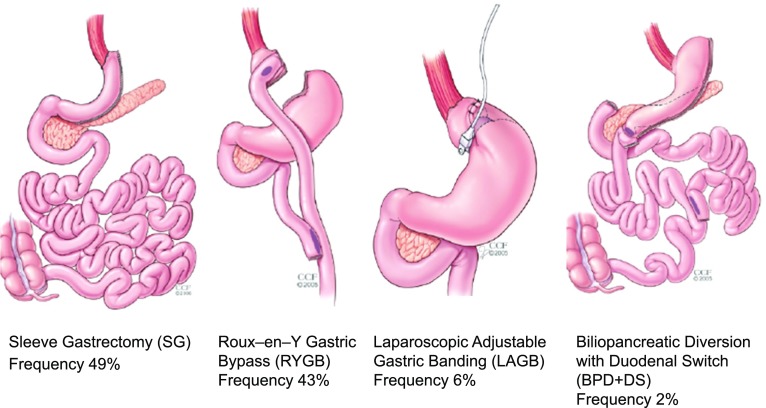
Gastrointestinal procedures intended to yield long-term weight loss in patients with severe obesity, otherwise known as bariatric surgery, have evolved since the 1950s and have become the most effective treatment for attaining significant and durable weight loss. Because metabolic and weight-related comorbidities, especially type 2 diabetes (T2D), are often improved or put into remission through weight loss or neuroendocrine mechanisms, the term metabolic surgery is rapidly replacing bariatric surgery. Other contributing mechanisms of diabetes control after surgery are also addressed in the articles from Batterham and Cummings (1) and Holst et al. (2), which appear in this issue of Diabetes Care. In general, metabolic operations have been historically thought to alter the gastrointestinal tract by 1) reducing stomach capacity, 2) rerouting nutrient flow, or 3) combining both concepts. Although these alterations may affect appetite, satiety, calorie absorption, and neuroendocrine pathways leading to weight loss, a complete understanding of weight-loss mechanisms after surgery is yet to be determined. Between 150,000–200,000 metabolic/bariatric procedures are performed annually in the U.S., and approximately 250,000 surgeries are performed outside the U.S. (3). The procedures along with frequency of use include sleeve gastrectomy (SG) (49%), Roux-en-Y gastric bypass (RYGB) (43%), laparoscopic adjustable gastric banding (LAGB) (6%), and biliopancreatic diversion with duodenal switch (BPD+DS) (2%) (4) (Fig. 1). SG has only recently replaced RYGB as the most common procedure worldwide, while LAGB has steadily declined in usage over the past 5–8 years (4). The development of laparoscopic approaches to all these metabolic procedures in the mid-1990s was a major advance, resulting in a significant reduction in perioperative morbidity and mortality. The original indications for bariatric surgery were based on BMI and were derived from the seminal National Institutes of Health (NIH) Consensus Conference in 1991, which considered surgery an option in patients with BMI ≥40 kg/m2 or with BMI ≥35 kg/m2 with significant obesity-related comorbidities (5). The relatively new 2013 American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society guidelines are similar and recommend that adults with BMI ≥35 kg/m2 and an obesity-related comorbidity, such as diabetes, who are motivated to lose weight be considered for referral to a bariatric surgeon (6). Such guidelines have focused primarily on surgery as a treatment for severe obesity; hence, the need for guidelines specific to T2D and related metabolic disorders.
Figure 1.
Common metabolic procedures and their frequency of use. Reprinted with permission from Cleveland Clinic Foundation.
2nd Diabetes Surgery Summit Rationale and Progress in Metabolic Surgery Since the 2007 Diabetes Surgery Summit
The primary goal of the 2nd Diabetes Surgery Summit (DSS-II), held on 28–30 September 2015 in London, England, was to review the new evidence regarding the safety and efficacy of metabolic surgery as a treatment for T2D since the 1st Diabetes Surgery Summit (DSS-I), held on 29–31 March 2007 in Rome, Italy, and then to modify the resultant treatment guidelines accordingly. In 2007, the existing evidence, summarized in an associated review article, was robust in terms of observational data supporting metabolic surgery but light in terms of randomized controlled trials (RCTs) (7). In fact, the only RCT at that time compared LAGB with conventional medical treatment of T2D and showed that LAGB was superior at 2-year follow-up with respect to remission of T2D (73% vs. 13%) (8). On the basis of the evidence available in 2007, the DSS-I Congress delegates concluded in the first guidelines for metabolic surgery that 1) metabolic surgery should be considered for the treatment of T2D in acceptable surgical candidates with BMI ≥35 kg/m2 who are inadequately controlled by lifestyle and medical therapy, 2) metabolic surgery may also be appropriate as a nonprimary alternative to treat inadequately controlled T2D in suitable surgical candidates with mild-to-moderate obesity (BMI 30–35 kg/m2), and 3) RCTs are strongly encouraged to assess the utility of gastrointestinal surgery to treat T2D (9). The American Diabetes Association (ADA) included metabolic surgery in their annual guidelines for the first time in 2009 but only for adults with BMI ≥35 kg/m2 and also advocated for RCTs comparing surgery with medical treatment of T2D (10). The 2011 International Diabetes Federation (IDF) position statement on metabolic surgery stated that metabolic surgery 1) should be an accepted option in people who have T2D and BMI ≥35 kg/m2 and 2) should also be considered as an alternative treatment option in people with T2D and BMI 30–35 kg/m2, or BMI 27.5–32.5 kg/m2 in Asian people and some other ethnicities, who have an increased risk when diabetes cannot be adequately controlled by optimal medical regimen (11). More RCTs comparing surgery with medical treatment were recommended by the IDF. This review article summarizes key evidence presented at the DSS-II, focusing on the effects of metabolic surgery on weight loss, glycemic control, remission of T2D, cardiovascular disease (CVD) risk reduction, and complications of surgery. Much emphasis will be placed on summarizing outcomes of new RCTs comparing surgery with medical management. An accompanying review article by Adams et al. (12) in this special collection in Diabetes Care will address the effect of metabolic surgery on hard clinical outcomes, including long-term mortality, cardiovascular events, and microvascular disease (retinopathy, nephropathy, and others).
Effect of Surgery on Weight Loss in Observational Studies
A large body of evidence (mostly observational) accumulated over three to four decades supports the contention that bariatric surgery is the most effective weight-loss intervention in terms of both magnitude of weight loss and durability. A major goal of metabolic procedures is the reduction of excess body fat and comorbidity improvement or remission. Weight loss is usually expressed as either percent weight loss ([weight loss in kg/initial weight in kg] × 100%) or percent excess weight loss (EWL) ([initial weight in kg − final weight in kg]/[initial weight − ideal body weight in kg] × 100%). Actual weight loss (in percent) is approximately half of EWL (13). A meta-analysis (136 studies) of mostly short-term (<5 years) weight-loss outcomes after >22,000 bariatric procedures demonstrated an overall mean EWL of 47.5% (95% CI 40.7 to −54.2) for patients who underwent LAGB, 61.6% (56.7 to −66.5) for RYGB, 68.2% (61.5 to −74.8) for gastroplasty, and 70.1% (66.3 to −73.9) for BPD (14). Weight loss for SG, the newest metabolic procedure, generally averages 50–55% EWL, which is intermediate between LAGB and RYGB (15). One of the largest and longest weight-loss studies is the Swedish Obese Subjects (SOS) study, a prospective study (99% follow-up rate) evaluating the long-term effects of bariatric surgery compared with nonsurgical weight management of severely obese (BMI >34 kg/m2) patients (16). At 20 years, the mean percent weight loss was 26% for gastric bypass, 18% for vertical banded gastroplasty, and 13% for gastric banding compared with 1% for control subjects. There is quite a bit of variation in weight-loss response, depending on procedure, with an expected percentage (2%–18%) regaining weight back to baseline; 18% for LAGB, 5% for SG, and 2% for RYGB. A rebound weight gain phenomenon occurs between short-term (meta-analysis) and long-term weight loss (SOS data) (17,18). By contrast, long-term medical (nonsurgical) weight loss rarely exceeds 5% (19).
Effect of Surgery on Glycemic Control, Remission, and CVD Risk Factors in Observational Studies
In addition to significant weight loss, investigators began reporting substantial improvement and in some cases remission of T2D in the late 1980s. Since then, multiple observational studies demonstrate significant, sustained improvements in T2D among patients with severe obesity (BMI ≥35 kg/m2) after weight-loss procedures. A meta-analysis, involving 19 studies (mostly observational) and 4,070 patients, reported an overall T2D resolution rate of 78% after bariatric surgery (20). Resolution was typically defined as becoming “nondiabetic” with normal HbA1c, without medications. Most of these studies, however, were retrospective, with follow-up of only 1–3 years on average, and varied by type of procedure. HbA1c typically improved from baseline by a minimum of 1%, up to 3%, following surgery, an effect rarely equaled by medical treatment alone. In the SOS study, the remission rate for T2D was 72% at 2 years and 36% at 10 years compared with 21% and 13%, respectively, for the nonsurgical control patients (P < 0.001) (21). Bariatric surgery was also markedly more effective than nonsurgical treatment in the prevention of T2D with a relative risk reduction of 78%. A recent systematic review of long-term cardiovascular risk factor reduction after bariatric surgery involved 73 studies and 19,543 patients (22). At a mean follow-up of 57.8 months, the average EWL for all procedures was 54% and remission/improvement was 63% for hypertension, 73% for T2D, and 65% for hyperlipidemia.
The evidence that these improvements in metabolic markers of disease, such as body weight, HbA1c, blood pressure, and lipids, after surgery actually translate to reduced macrovascular and microvascular events is mounting. Adams et al. (12) addresses this subject in more detail in their article. In brief, 12 cohort-matched, nonrandomized studies comparing bariatric surgery with nonsurgical control subjects have been recently reviewed, and they broadly support a cardiovascular event and all-cause mortality benefit conferred by bariatric surgery (23). All but two of these studies support a lower CVD event rate and all-cause mortality rate among patients who had undergone bariatric surgery. The two negative studies were of relative short follow-up (6 years), perhaps an insufficient time to demonstrate a mortality benefit (24,25). The study by Maciejewski et al. (25) involved mostly higher-risk, male veterans, who were later determined after longer follow-up to demonstrate a 42% reduction in mortality at 10 years with bariatric surgery as compared with medical therapy (26). The SOS study has the longest outcome follow-up (median of 14.7 years). CVD mortality in the surgical group was lower than in control subjects (adjusted hazard ratio 0.47 [95% CI 0.29–0.76]; P = 0.002), despite a greater prevalence of smoking and higher baseline weights and blood pressures in the surgical cohort (16). Few, mostly retrospective, studies have evaluated the effect of metabolic surgery on the progression of microvascular disease, such as retinopathy, nephropathy, and neuropathy, in T2D. The potential of reversal or reduced development of nephropathy after bariatric surgery has been reported (27,28). In the SOS study, for patients with T2D, surgery was associated with a 50% reduction in microvascular complications (29). After 15 years of follow-up, the cumulative incidence of microvascular complications was 41.8 per 1,000 person-years (95% CI 35.3–49.5) for control subjects and 20.6 per 1,000 person-years (95% CI 17.0–24.9) in the surgery group (hazard ratio 0.44 [95% CI 0.34–0.56]; P < 0.001). These observational studies collectively suggest that bariatric surgery may significantly improve glycemic control and cardiovascular risk factors and reduce long-term morbidity and mortality of T2D compared with medical management alone, yet they have not been validated by RCTs.
RCTs of Metabolic Surgery
The most significant advance in metabolic surgery since the DSS-I has been the completion and publication of 11 RCTs comparing metabolic surgery with medical treatment of T2D (Table 1) (8,30–43). All of these RCTs included patients with T2D and obesity totaling 794 randomized subjects (n = 38 for smallest study, n = 150 for largest study) with follow-up from 6 months to 5 years (6 studies with ≥2 years, 3 studies with ≥3 years) with approximately 10% dropout overall. Few crossovers (2) from the medical treatment group to surgery have been reported thus far (33) and they were left out of the 5-year analysis. The surgical procedures evaluated included RYGB (7 studies), LAGB (4 studies), BPD (1 study), and SG (1 study). Eight studies compared one procedure (two arms) with medical treatment of T2D, and 3 compared two procedures (three arms). The control groups varied significantly in that some followed conventional treatment guidelines as per the ADA (8,33), others involved intensive lifestyle interventions as per Look AHEAD (Action for Health in Diabetes) (38), and still others involved intensive pharmacotherapy (30). The remaining studies were a hybrid of all three strategies. The severity of T2D among the studies also varied significantly from mild (mean HbA1c 7.7%, <2 years from onset, no insulin) (8) to advanced (mean HbA1c 9.3%, 8.3 years of duration, 48% on insulin) (30). The BMI ranged from 25 to 53 kg/m2 with 9 of 11 studies including patients with BMI ≤35 kg/m2 (45% of total). Demographics in terms of age, sex, and ethnic background were similar, although 2 studies (34,36) included a significant number of Asian patients. For most of the studies, the primary or secondary end point was the success rate of reaching remission, defined as reaching an HbA1c target (≤6.0%–6.5%) without requiring diabetes medications. Schauer et al. (30) had a primary end point of HbA1c of ≤6% with or without medications, and the others had various end points (e.g., Wentworth et al. [40]: fasting blood glucose <7.0 mmol/L). Ikramuddin et al. (34) uniquely used a composite primary end point based on the ADA 2009 guidelines defined as the success rate of reaching an HbA1c<7.0%, LDL cholesterol <100 mg/dL, and systolic blood pressure <130 mmHg.
Table 1.
Metabolic surgery RCTs for T2D (n = 794)
| Study | BMI (kg/m2), % of patients | Design | No. of patients randomized | Follow-up (months) | Remission criteria* | Outcome (remission or change in HbA1c) |
|---|---|---|---|---|---|---|
| Dixon (8) | <35, 22% | LAGB vs. control | 60 | 24 | HbA1c <6.2% | 73% vs. 13%, P < 0.001 |
| Schauer (30,31) | <35, 36% | RYGB vs. SG vs. control | 150 | 36 | HbA1c ≤6.0% | 35% vs. 20% vs. 0, P = 0.002 |
| Mingrone (32,33) | >35, 100% | RYGB vs. BPD vs. control | 60 | 60 | HbA1c ≤6.5% | 42% vs. 68% vs. 0, P = 0.003 |
| Ikramuddin (34,35) | <35, 59% | RYGB vs. control | 120 | 24 | HbA1c <6% | 44% vs. 9%, P < 0.001 |
| Liang (36) | <35, 100% | RYGB vs. control | 101 | 12 | HbA1c <6.5%** | 90% vs. 0 vs. 0, P < 0.0001 |
| Halperin (37) | <35, 34% | RYGB vs. control | 38 | 12 | HbA1c <6.5% | 58% vs. 16%, P = 0.03 |
| Courcoulas (38,39) | <35, 43% | RYGB vs. LAGB vs. control | 69 | 36 | HbA1c <6.5% | 40% vs. 29% vs. 0, P = 0.004 |
| Wentworth (40) | ≤30, 100% | LAGB vs. control | 51 | 24 | Fasting blood glucose <7.0 mmol/L | 52% vs. 8%, P = 0.001 |
| Parikh (41) | <35, 100% | Bariatric surgery (RYGB, LAGB, SG) vs. control | 57 | 6 | HbA1c <6.5% | 65% vs. 0, P = 0.0001 |
| Ding (42) | <35, 34% | LAGB vs. control | 45 | 12 | HbA1c <6.5%*** | 33% vs. 23%, P = 0.46 |
| Cummings (43) | <35, 25% | RYGB vs. control | 43 | 12 | HbA1c <6.0% | 60% vs. 5.9%, P = 0.002 |
*Remission was a primary or secondary end point. Reaching HbA1c value without diabetes medication, unless otherwise specified.
**Remission not precisely defined, HbA1c <6.5% by extrapolation.
***On or off diabetes medications.
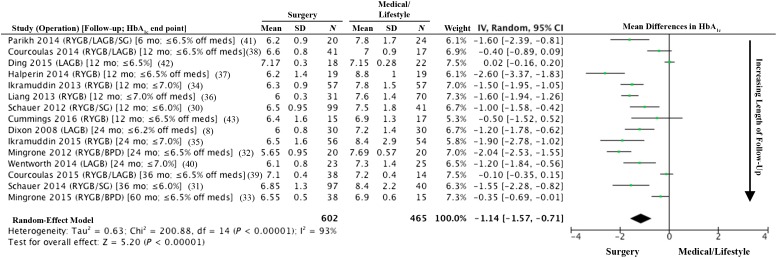
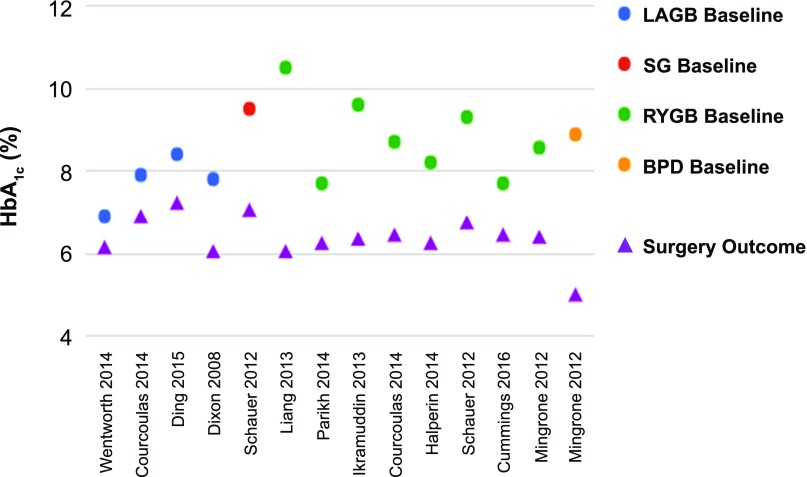
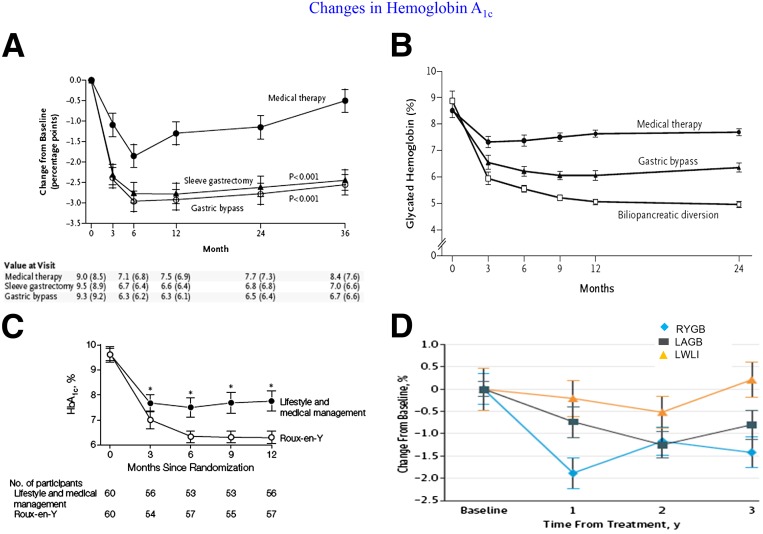
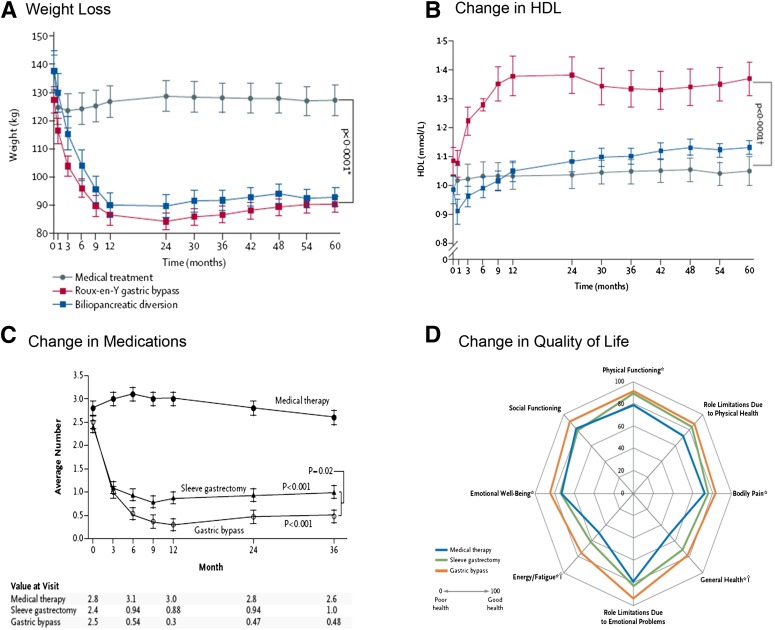
Despite the variability in study design and patient characteristics of the 11 RCTs, there was a remarkable degree of consistency in terms of major outcomes favoring surgery. With the exception of 1 study (42), all others (10 of 11) showed that surgery was superior to medical treatment with respect to reaching the primary end point (P < 0.05 for all) (Fig. 2). In the study by Ding et al. (42), diabetes remission for LAGB and medical treatment was 33% and 23%, respectively (P = 0.46). This negative result might be explained by considering that LAGB is less effective than the other metabolic procedures and that this study involved patients with advanced T2D (HbA1c 8.2% ± 1.2%, 40% on insulin) who might have a reduced response to treatment due to reduced β-cell function. Typically, surgery resulted in a decrease in HbA1c by 2%–3.5%, whereas medical weight loss resulted in 1%–1.5% decrease in HbA1c (Figs. 3 and 4). Most of these studies showed superiority of surgery over medical treatment in achieving secondary end points such as weight loss, change in HbA1c from baseline, remission of metabolic syndrome, reduction in diabetes and cardiovascular medications, and improvement in triglycerides, HDL, and quality of life (Fig. 5). Results were mixed in terms of improvements in systolic and diastolic blood pressure (34,39) and LDL cholesterol (33) after surgery compared with medical treatment, but many studies did show a corresponding reduction in medication usage. Furthermore, in many of the studies, blood pressure and LDL cholesterol were in good control at study entry, perhaps because patients were receiving effective medical treatment. Mingrone et al. (33) had the longest follow-up (5 years) and showed superior and durable weight loss and glycemic control (remission) with both RYGB and BPD compared with medical therapy (Fig. 6). Among the RCTs, the most common predictors of diabetes remission included duration of diabetes, weight loss, requirement for insulin, and disease status (HbA1c) (31,33,35).
Figure 2.
Forest plot of mean differences (MDs) of %HbA1c serum levels after bariatric/metabolic surgery compared with medical/lifestyle treatments in published RCTs. Data are arranged in order of ascending time to follow-up. Study duration and HbA1c end point thresholds are shown in brackets in column 1, where “off meds” indicates a threshold achieved off all diabetes medications; otherwise, end points represent HbA1c thresholds achieved with or without such medications. Negative MDs denote lower HbA1c levels following surgery than medical/lifestyle treatment. MDs for each study are shown as the MD with its 95% CI.
Figure 3.
Change in HbA1c after LAGB, RYGB, SG, and BPD in 11 RCTs.
Figure 4.
Change in HbA1c after surgical vs. medical treatment of T2D in the studies by Schauer et al. (31) (A), Mingrone et al. (32) (B), Ikramuddin et al. (34) (C), and Courcoulas et al. (39) (D). LWLI, lifestyle weight-loss intervention; y, years. Reprinted with permission from the four studies.
Figure 5.
Other secondary end points favoring surgery over medical treatment. A: Weight loss in the study by Mingrone et al. (33). *From ANOVA comparison. B: Change in HDL (33). †From nonparametric tests. C: Change in medications in the study by Schauer et al. (31). D: Change in quality of life (31). *P < 0.05 for the comparison between the gastric bypass group and the medical therapy group; †P < 0.05 for the comparison between the sleeve gastrectomy group and the medical therapy group. Reprinted with permission from the two studies.
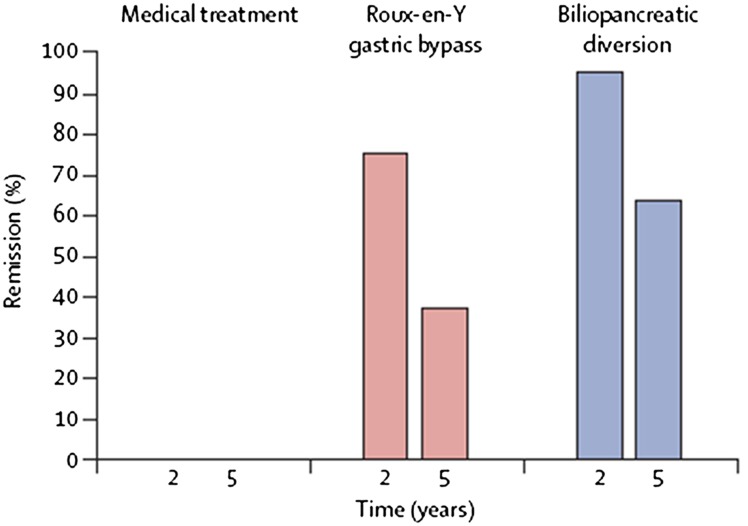
Figure 6.
Remission at 5 years in the study by Mingrone et al. (33).
None of these 11 RCTs were powered sufficiently to detect differences in macrovascular or microvascular events, especially at relatively short follow-up, and no such differences have been detected thus far. However, improvement in albuminuria after surgery (RYGB, 64% decrease, P = 0.04) was noted by Schauer et al. (31) in the Surgical Therapy And Medications Potentially Eradicate Diabetes Efficiently (STAMPEDE) trial at 3 years despite a reduction in renin-angiotensin-aldosterone system blockers, suggesting that bariatric surgery may have a role in the prevention of further parenchymal damage. In addition, the STAMPEDE trial demonstrated that bariatric surgery (RYGB or SG) did not appear to worsen or improve retinopathy outcomes at 2 years compared with intensive medical management (P = 0.84), and a majority, 86.5%, of patients within all treatment groups had no change in retinopathy scoring (44). Additionally, there was no significant change in LogMAR visual acuity from baseline among the treatment arms (P > 0.05), as the mean baseline and 2-year visual acuity were the same in all three groups (LogMAR 0, Snellen equivalent 20/20).
Complications of Metabolic Surgery
The risks of metabolic surgery must be assessed along with the aforementioned benefits. A large meta-analysis of case series of bariatric surgery reported an overall 30-day postoperative mortality of 0.28% (n = 84,931) and total mortality from 30 days to 2 years was 0.35% (n = 19,928) (45). The NIH-supported Longitudinal Assessment of Bariatric Surgery (LABS) study subsequently reported a similarly low 30-day mortality rate (0.3%) among 4,776 patients and a 4.3% incidence of major adverse events in the early postoperative period (46). To address the perioperative risks in patients with T2D, a recent study from the American College of Surgeons National Surgical Quality Improvement Program (NSQIP) involving more than 65,000 patients found for RYGB major perioperative morbidity and mortality rates of 3.4% and 0.3%, respectively, comparable to common, low-risk procedures such as laparoscopic cholecystectomy (3.7% and 0.7%, respectively) and appendectomy (4.5% and 0.5%, respectively) (47). RYGB had a much lower risk than the morbidity and mortality of colectomy (12.0% and 1.7%, respectively).
A summary of early and late complications after bariatric surgery is shown in Table 2. Cardiopulmonary complications, such as myocardial infarction and pulmonary embolism, although rare (<1%), are the major causes of mortality, representing 70% of all perioperative deaths (46). The most serious surgical complication after RYGB is anastomotic leakage, with an incidence ranging from 0.1% to 5.6%, followed by bowel obstruction (0.5%–2%) and marginal ulcers (1%–5%) (48,49). Leakage may potentially lead to life-threatening peritonitis. Staple line leakage after SG is similarly in the range of 1%–5% (15). For BPD+DS, perioperative complications are similar to RYGB. LAGB is a safe procedure with 0.3% or less mortality rate (46). However, late complications such as band slippage, erosion, migration, port infection, and gastroesophageal perforations are well documented and occur in about 20% of patients (50). In addition, long-term weight-loss failure rates of over 50% have been reported, and this has led to a significant increase in revisions of LAGB to RYGB or SG (51). Patients at higher risk for complications are males, smokers, and those with higher BMI, older age, multiple comorbidities, or prior revisional operations (46).
Table 2.
Complications of metabolic surgery
| Complications | Frequency (%) |
|---|---|
| Sepsis from anastomotic leak | 0.1–5.6 |
| Hemorrhage | 1–4 |
| Cardiopulmonary events | <1 |
| Thromboembolic disease | 0.34 |
| Death | 0.1–0.3 |
| Late complications for LAGB | |
| Band slippage | 15 |
| Leakage | 2–5 |
| Erosion | 1–2 |
| Late complications of bypass procedures | |
| Anastomotic strictures | 1–5 |
| Marginal ulcers | 1–5 |
| Bowel obstructions | 0.5–2 |
| Kidney stones | NK |
| Metabolic bone disease | NK |
| Alcohol use disorder | NK |
| Micronutrient and macronutrient deficiencies from RYGB 2–3 years postoperative | |
| Iron deficiency | 45–52 |
| Vitamin B12 deficiency | 8–37 |
| Calcium deficiency | 10 |
| Vitamin D deficiency | 51 |
| Fat-soluble vitamin deficiencies (A, D, E, and K) and protein calorie malnutrition from BPD+DS procedures | 1–5 |
NK, not known.
In assessing nutritional deficiencies after bariatric surgery, it is important to note that there is a high prevalence of nutritional deficiencies (35%–80%) in patients with severe obesity seeking bariatric surgery. In one study, preoperative deficiencies were common: vitamin A (11%), vitamin B12 (13%), vitamin D (40%), zinc (30%), iron (16%), ferritin (9%), selenium (58%), and folate (6%) (52). Postoperative deficiencies are typically associated with diminished nutrient intake or the malabsorptive effect of bariatric procedures and are more commonly seen after BPD+DS and RYGB and are less commonly seen after SG and LAGB. Anemia (mild) is common after RYGB (15%–20%) and iron deficiency ranges from 17% to 50%; the etiology is believed to be multifactorial in nature including reduced iron and vitamin B12 absorption as well as a high rate of preexisting iron deficiency anemia in premenopausal patients (53). Deficiencies in trace minerals (selenium, zinc, and copper) and vitamins (B12, B1, A, E, D, and K) can be observed after bariatric procedures, specifically after BPD+DS (54). With appropriate vitamin, iron, and calcium supplementation, such deficits can be prevented or corrected (55). A decrease in bone mineral density (14% in proximal femur) has been determined after bariatric surgery (56). Reduced mechanical loading, micronutrient deficiency, and malabsorption, along with neurohormonal alterations, are potential underlying mechanisms that explain these observed postsurgical changes in bone density. Very limited data on fracture and osteoporosis incidence raise questions about whether marked postbariatric surgery bone loss is clinically relevant or a functional adaptation to skeletal unloading. Severe calcium and vitamin D deficiencies have been described after the extreme malabsorptive procedures BPD+DS and very long limb RYGB, leading to decreased bone mineral density and osteoporosis. Protein malnutrition can also occur after these malabsorptive operations and lifelong monitoring for nutritional complications is mandatory following these procedures (57).
Other late complications following bariatric surgery that are somewhat controversial as to their incidence and significance but worth mentioning include kidney stones, alcohol abuse, and depression/suicide. The literature suggests that kidney stone formation may increase after bariatric surgery, particularly bypass operations, but the relative increase is not well documented. One study of patients after RYGB (n = 4,690) used insurance claims data to note a significantly higher prevalence of kidney stones (7.5% compared with 4.6%) in obese control subjects (58). The underlying mechanisms of kidney stone formation following bariatric surgery are complex and include hyperoxaluria, hypocitraturia, and abnormally acid urine (59). RYGB has been associated with the risk of developing an alcohol use disorder, although the degree of risk is not clear. In a recent systematic review, the prevalence of postoperative alcohol use was higher among patients with preoperative history of alcohol use than those without. Postoperative prevalence of alcohol use ranged from 7.6% to 11.8% (60). Paradoxically, although bariatric surgery has been shown to significantly decrease depression (61), some studies suggest that a slight increase in suicide may occur after bariatric surgery (62), whereas others do not (15). A recent review concluded that although the data are limited based only on earlier anecdotal reports and more recent epidemiological data, the available data suggest an increased risk of suicide, although uncommon, after bariatric surgery (63).
Although the 11 RCTs reported above were not powered to detect differences in treatment-related complications, complication assessment may be instructive. The most common significant surgical complications were anemia (15%), reoperation (8%), and gastrointestinal (5–10%). There were no perioperative deaths or cardiovascular events in the 794 patients. Among the surgical procedures, BPD had the highest rate of nutrition-related complications (hypoalbuminemia 16%, osteopenia 16%, osteoporosis 5%, renal calculus 11%), whereas corresponding rates in the medically treated groups were negligible except for osteopenia (7%) (31,33). In the medical control group, greater than 5% weight gain (16%) was among the most common adverse event (31). Investigators did report, in general, challenges with medication compliance and discontinuing medications due to side effects. Hypoglycemia (mild) was common in both surgical and medical patients, but when reported, there were no differences between treatment groups (31,33,34).
Conclusions
Metabolic surgery can reverse or improve T2D. There is now evidence supporting decreases in short- and medium-term CVD. Until recently, these data were derived from observational studies only. Now there are 11 RCTs with 794 patients comparing metabolic surgery with medical treatment of T2D that show a remarkable degree of consistency in the superiority of surgery (except LAGB) in achieving glycemic control. Remarkably, some patients achieved complete remission without reliance on medications. Many surgical patients also had improvements in cardiovascular risk factors, such as weight loss, lipids, blood pressure, and quality of life, compared with medical patients. Benefits of surgery should be weighed against short- and long-term complications, which are best managed by a long-term multidisciplinary effort. Early perioperative morbidity (4–5%) and mortality (0.3%) following metabolic surgery is relatively low compared with other abdominal procedures, such as cholecystectomy. Metabolic surgery may be particularly suitable for patients with T2D and severe obesity (BMI ≥35 kg/m2), as these patients may benefit from obesity comorbidity improvement and significantly improved glycemic control compared with patients treated with medical therapy alone. Patients with class I obesity (BMI 30–35 kg/m2) may also benefit from bariatric surgery, especially if their T2D is poorly controlled with optimal medical therapy. Taken together, these data highlight how metabolic surgery can result in significant glycemic improvement in nearly all patients with T2D and result in long-term remission in some patients. In addition, significant weight loss, medication reduction, quality-of-life improvement, and CVD risk factor improvement have been shown to consistently occur after metabolic surgery. The durability of these metabolic improvements, particularly from the RCT literature, remains to be determined and represents an important future area of research.
Article Information
Duality of Interest. P.R.S. receives grants from NIH, Ethicon, Medtronic, and Pacira; receives consulting fees from Ethicon, The Medicines Company, and SurgiQuest; and holds stock from SurgiQuest. No other potential conflicts of interest relevant to this article were reported.
Footnotes
References
- 1.Batterham RL, Cummings DE. Mechanisms of diabetes improvement following bariatric/metabolic surgery. Diabetes Care 2016;39:893–901 [DOI] [PMC free article] [PubMed]
- 2.Holst JJ, Gribble F, Horowitz M, Rayner CK. Roles of the gut in glucose homeostasis. Diabetes Care 2016;39:884–892 [DOI] [PubMed] [Google Scholar]
- 3.Angrisani L, Santonicola A, Iovino P, Formisano G, Buchwald H, Scopinaro N. Bariatric surgery worldwide 2013. Obes Surg 2015;25:1822–1832 [DOI] [PubMed] [Google Scholar]
- 4.Khorgami Z, Andalib A, Corcelles R, Aminian A, Brethauer S, Schauer P. Recent national trends in the surgical treatment of obesity: sleeve gastrectomy dominates. Surg Obes Relat Dis 2015;11:S6–S8 [Google Scholar]
- 5.Consensus Development Conference Panel NIH conference. Gastrointestinal surgery for severe obesity. Ann Intern Med 1991;115:956–961 [PubMed] [Google Scholar]
- 6.Jensen MD, Ryan DH, Apovian CM, et al.; American College of Cardiology/American Heart Association Task Force on Practice Guidelines; The Obesity Society. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society [published correction appears in J Am Coll Cardiol 2014;63:3029–3030]. J Am Coll Cardiol 2014;63:2985–3023 [DOI] [PubMed] [Google Scholar]
- 7.Rubino F, Schauer PR, Kaplan LM, Cummings DE. Metabolic surgery to treat type 2 diabetes: clinical outcomes and mechanisms of action. Annu Rev Med 2010;61:393–411 [DOI] [PubMed] [Google Scholar]
- 8.Dixon JB, O’Brien PE, Playfair J, et al. . Adjustable gastric banding and conventional therapy for type 2 diabetes: a randomized controlled trial. JAMA 2008;299:316–323 [DOI] [PubMed] [Google Scholar]
- 9.Rubino F, Kaplan LM, Schauer PR, Cummings DE; Diabetes Surgery Summit Delegates . The Diabetes Surgery Summit consensus conference: recommendations for the evaluation and use of gastrointestinal surgery to treat type 2 diabetes mellitus. Ann Surg 2010;251:399–405 [DOI] [PubMed] [Google Scholar]
- 10.American Diabetes Association. Standards of medical care in diabetes—2009. Diabetes Care 2009;32(Suppl. 1):S13–S61 [DOI] [PMC free article] [PubMed]
- 11.Dixon JB, Zimmet P, Alberti KG, Rubino F; International Diabetes Federation Taskforce on Epidemiology and Prevention . Bariatric surgery: an IDF statement for obese type 2 diabetes. Diabet Med 2011;28:628–642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Adams TD, Arterburn DE, Nathan DM, Eckel RH. Clinical outcomes of metabolic surgery: microvascular and macrovascular complications. Diabetes Care 2016;39:912–923 [DOI] [PMC free article] [PubMed]
- 13.Belle SH, Berk PD, Courcoulas AP, et al.; LABS Consortium Weight Change Writing Group . Reporting weight change: standardized reporting accounting for baseline weight. Surg Obes Relat Dis 2013;9:782–789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Buchwald H, Avidor Y, Braunwald E, et al. . Bariatric surgery: a systematic review and meta-analysis. JAMA 2004;292:1724–1737 [DOI] [PubMed] [Google Scholar]
- 15.Brethauer SA, Hammel JP, Schauer PR. Systematic review of sleeve gastrectomy as staging and primary bariatric procedure. Surg Obes Relat Dis 2009;5:469–475 [DOI] [PubMed] [Google Scholar]
- 16.Sjöström L, Peltonen M, Jacobson P, et al. . Bariatric surgery and long-term cardiovascular events. JAMA 2012;307:56–65 [DOI] [PubMed] [Google Scholar]
- 17.Courcoulas AP, Christian NJ, Belle SH, et al.; Longitudinal Assessment of Bariatric Surgery (LABS) Consortium . Weight change and health outcomes at 3 years after bariatric surgery among individuals with severe obesity. JAMA 2013;310:2416–2425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Manning S, Pucci A, Carter NC, et al. . Early postoperative weight loss predicts maximal weight loss after sleeve gastrectomy and Roux-en-Y gastric bypass. Surg Endosc 2015;29:1484–1491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wing RR, Bolin P, Brancati FL, et al.; Look AHEAD Research Group . Cardiovascular effects of intensive lifestyle intervention in type 2 diabetes. N Engl J Med 2013;369:145–154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Buchwald H, Estok R, Fahrbach K, et al. . Weight and type 2 diabetes after bariatric surgery: systematic review and meta-analysis. Am J Med 2009;122:248–256.e5 [DOI] [PubMed] [Google Scholar]
- 21.Sjöström L, Lindroos AK, Peltonen M, et al.; Swedish Obese Subjects Study Scientific Group . Lifestyle, diabetes, and cardiovascular risk factors 10 years after bariatric surgery. N Engl J Med 2004;351:2683–2693 [DOI] [PubMed] [Google Scholar]
- 22.Vest AR, Heneghan HM, Agarwal S, Schauer PR, Young JB. Bariatric surgery and cardiovascular outcomes: a systematic review. Heart 2012;98:1763–1777 [DOI] [PubMed] [Google Scholar]
- 23.Vest AR, Heneghan HM, Schauer PR, Young JB. Surgical management of obesity and the relationship to cardiovascular disease. Circulation 2013;127:945–959 [DOI] [PubMed] [Google Scholar]
- 24.Adams TD, Davidson LE, Litwin SE, et al. . Health benefits of gastric bypass surgery after 6 years. JAMA 2012;308:1122–1131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maciejewski ML, Livingston EH, Smith VA, et al. . Survival among high-risk patients after bariatric surgery. JAMA 2011;305:2419–2426 [DOI] [PubMed] [Google Scholar]
- 26.Arterburn DE, Olsen MK, Smith VA, et al. . Association between bariatric surgery and long-term survival. JAMA 2015;313:62–70 [DOI] [PubMed] [Google Scholar]
- 27.Iaconelli A, Panunzi S, De Gaetano A, et al. . Effects of bilio-pancreatic diversion on diabetic complications: a 10-year follow-up. Diabetes Care 2011;34:561–567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brethauer SA, Aminian A, Romero-Talamás H, et al. . Can diabetes be surgically cured? Long-term metabolic effects of bariatric surgery in obese patients with type 2 diabetes mellitus. Ann Surg 2013;258:628–636; discussion 636–637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sjöström L, Peltonen M, Jacobson P, et al. . Association of bariatric surgery with long-term remission of type 2 diabetes and with microvascular and macrovascular complications. JAMA 2014;311:2297–2304 [DOI] [PubMed] [Google Scholar]
- 30.Schauer PR, Kashyap SR, Wolski K, et al. . Bariatric surgery versus intensive medical therapy in obese patients with diabetes. N Engl J Med 2012;366:1567–1576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schauer PR, Bhatt DL, Kirwan JP, et al.; STAMPEDE Investigators . Bariatric surgery versus intensive medical therapy for diabetes--3-year outcomes. N Engl J Med 2014;370:2002–2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mingrone G, Panunzi S, De Gaetano A, et al. . Bariatric surgery versus conventional medical therapy for type 2 diabetes. N Engl J Med 2012;366:1577–1585 [DOI] [PubMed] [Google Scholar]
- 33.Mingrone G, Panunzi S, De Gaetano A, et al. . Bariatric-metabolic surgery versus conventional medical treatment in obese patients with type 2 diabetes: 5 year follow-up of an open-label, single-centre, randomised controlled trial. Lancet 2015;386:964–973 [DOI] [PubMed] [Google Scholar]
- 34.Ikramuddin S, Korner J, Lee W-J, et al. . Roux-en-Y gastric bypass vs intensive medical management for the control of type 2 diabetes, hypertension, and hyperlipidemia: the Diabetes Surgery Study randomized clinical trial. JAMA 2013;309:2240–2249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ikramuddin S, Billington CJ, Lee WJ, et al. . Roux-en-Y gastric bypass for diabetes (the Diabetes Surgery Study): 2-year outcomes of a 5-year, randomised, controlled trial. Lancet Diabetes Endocrinol 2015;3:413–422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liang Z, Wu Q, Chen B, Yu P, Zhao H, Ouyang X. Effect of laparoscopic Roux-en-Y gastric bypass surgery on type 2 diabetes mellitus with hypertension: a randomized controlled trial. Diabetes Res Clin Pract 2013;101:50–56 [DOI] [PubMed] [Google Scholar]
- 37.Halperin F, Ding SA, Simonson DC, et al. . Roux-en-Y gastric bypass surgery or lifestyle with intensive medical management in patients with type 2 diabetes: feasibility and 1-year results of a randomized clinical trial. JAMA Surg 2014;149:716–726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Courcoulas AP, Goodpaster BH, Eagleton JK, et al. . Surgical vs medical treatments for type 2 diabetes mellitus: a randomized clinical trial. JAMA Surg 2014;149:707–715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Courcoulas AP, Belle SH, Neiberg RH, et al. . Three-year outcomes of bariatric surgery vs lifestyle intervention for type 2 diabetes mellitus treatment: a randomized clinical trial. JAMA Surg 2015;150:931–940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wentworth JM, Playfair J, Laurie C, et al. . Multidisciplinary diabetes care with and without bariatric surgery in overweight people: a randomised controlled trial. Lancet Diabetes Endocrinol 2014;2:545–552 [DOI] [PubMed] [Google Scholar]
- 41.Parikh M, Chung M, Sheth S, et al. . Randomized pilot trial of bariatric surgery versus intensive medical weight management on diabetes remission in type 2 diabetic patients who do not meet NIH criteria for surgery and the role of soluble RAGE as a novel biomarker of success. Ann Surg 2014;260:617–622; discussion 622–624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ding SA, Simonson DC, Wewalka M, et al. . Adjustable gastric band surgery or medical management in patients with type 2 diabetes: a randomized clinical trial. J Clin Endocrinol Metab 2015;100:2546–2556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cummings DE, Arterburn DE, Westbrook EO, et al. . Gastric bypass surgery vs intensive lifestyle and medical intervention for type 2 diabetes: the CROSSROADS randomised controlled trial. Diabetologia 2016;59:945–953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Singh RP, Gans R, Kashyap SR, et al. . Effect of bariatric surgery versus intensive medical management on diabetic ophthalmic outcomes. Diabetes Care 2015;38:e32–e33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Buchwald H, Estok R, Fahrbach K, Banel D, Sledge I. Trends in mortality in bariatric surgery: a systematic review and meta-analysis. Surgery 2007;142:621–632; discussion 632–635 [DOI] [PubMed] [Google Scholar]
- 46.Flum DR, Belle SH, King WC, et al.; Longitudinal Assessment of Bariatric Surgery (LABS) Consortium . Perioperative safety in the Longitudinal Assessment of Bariatric Surgery. N Engl J Med 2009;361:445–454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Aminian A, Brethauer SA, Kirwan JP, Kashyap SR, Burguera B, Schauer PR. How safe is metabolic/diabetes surgery? Diabetes Obes Metab 2015;17:198–201 [DOI] [PubMed] [Google Scholar]
- 48.Thodiyil PA, Yenumula P, Rogula T, et al. . Selective nonoperative management of leaks after gastric bypass: lessons learned from 2675 consecutive patients. Ann Surg 2008;248:782–792 [DOI] [PubMed] [Google Scholar]
- 49.Rogula T, Yenumula PR, Schauer PR. A complication of Roux-en-Y gastric bypass: intestinal obstruction. Surg Endosc 2007;21:1914–1918 [DOI] [PubMed] [Google Scholar]
- 50.Thornton CM, Rozen WM, So D, Kaplan ED, Wilkinson S. Reducing band slippage in laparoscopic adjustable gastric banding: the mesh plication pars flaccida technique. Obes Surg 2009;19:1702–1706 [DOI] [PubMed] [Google Scholar]
- 51.Himpens J, Cadière G-B, Bazi M, Vouche M, Cadière B, Dapri G. Long-term outcomes of laparoscopic adjustable gastric banding. Arch Surg 2011;146:802–807 [DOI] [PubMed] [Google Scholar]
- 52.Madan AK, Orth WS, Tichansky DS, Ternovits CA. Vitamin and trace mineral levels after laparoscopic gastric bypass. Obes Surg 2006;16:603–606 [DOI] [PubMed] [Google Scholar]
- 53.Love AL, Billett HH. Obesity, bariatric surgery, and iron deficiency: true, true, true and related. Am J Hematol 2008;83:403–409 [DOI] [PubMed] [Google Scholar]
- 54.Shankar P, Boylan M, Sriram K. Micronutrient deficiencies after bariatric surgery. Nutrition 2010;26:1031–1037 [DOI] [PubMed] [Google Scholar]
- 55.Gong K, Gagner M, Pomp A, Almahmeed T, Bardaro SJ. Micronutrient deficiencies after laparoscopic gastric bypass: recommendations. Obes Surg 2008;18:1062–1066 [DOI] [PubMed] [Google Scholar]
- 56.Scibora LM. Skeletal effects of bariatric surgery: examining bone loss, potential mechanisms and clinical relevance. Diabetes Obes Metab 2014;16:1204–1213 [DOI] [PubMed] [Google Scholar]
- 57.Baptista V, Wassef W. Bariatric procedures: an update on techniques, outcomes and complications. Curr Opin Gastroenterol 2013;29:684–693 [DOI] [PubMed] [Google Scholar]
- 58.Matlaga BR, Shore AD, Magnuson T, Clark JM, Johns R, Makary MA. Effect of gastric bypass surgery on kidney stone disease. J Urol 2009;181:2573–2577 [DOI] [PubMed] [Google Scholar]
- 59.Sakhaee K, Poindexter J, Aguirre C. The effects of bariatric surgery on bone and nephrolithiasis. Bone 2016;84:1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Li L, Wu LT. Substance use after bariatric surgery: a review. J Psychiatr Res 2016;76:16–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ayloo S, Thompson K, Choudhury N, Sheriffdeen R. Correlation between the Beck Depression Inventory and bariatric surgical procedures. Surg Obes Relat Dis 2015;11:637–642 [DOI] [PubMed] [Google Scholar]
- 62.Adams TD, Gress RE, Smith SC, et al. . Long-term mortality after gastric bypass surgery. N Engl J Med 2007;357:753–761 [DOI] [PubMed] [Google Scholar]
- 63.Mitchell JE, Crosby R, de Zwaan M, et al. . Possible risk factors for increased suicide following bariatric surgery. Obesity (Silver Spring) 2013;21:665–672 [DOI] [PMC free article] [PubMed] [Google Scholar]