Fig. 2.
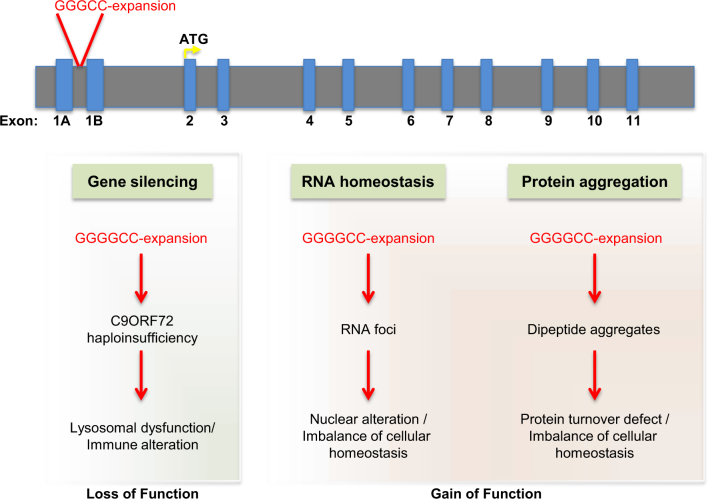
C9ORF72 associates with multiple cellular pathways relevant to ALS/FTLD. A schematic representation of the genomic structure of C9ORF72, showing the location of the (GGGGCC) hexanucleotide expansion in the intronic region between two alternative non-coding first exons. This expansion leads to three observable phenotypes. One is C9ORF72 loss-of-function leading to a blockade of lysosomal function and an alteration in immune function. Based on the RNA expression profile of this gene, this haploinsufficiency phenotype primarily affects microglia in the CNS. In addition, there are two other phenotypes observed in both C9ORF72 ALS/FTLD patients and in animal models of the disease. The (GGGGCC) hexanucleotide expansion leads to the formation on RNA foci, which results in the mislocalization and sequestration of RNA-binding proteins causing nuclear and cellular dysfunction. Finally, through repeat-associated non-ATG-dependent translation, (GGGGCC) hexanucleotide repeats can produce five different types of dipeptide repeat sequences, depending on the direction (sense and anti-sense) and frame of translation (GA, GR, GP, PR, and PA). These aberrant species lead to protein aggregation, toxicity, lysosomal blockade, and overall cellular dysfunction. This most common genetic cause of ALS/FTLD illustrates the potential contribution of disruptions to multiple cellular pathways in various cell types to disease