Abstract
Prior research suggests that several endogenous hormones in premenopausal women are associated with breast cancer risk; however, few studies have evaluated associations of endogenous hormones with mammographic density (MD) in premenopausal women. We conducted a cross-sectional study of plasma hormone levels in relation to MD among 634 cancer-free premenopausal women in the Nurses’ Health Study II. We measured percent MD from screening mammograms using a computer-assisted method. We assayed estradiol, estrone, and estrone sulfate in blood samples timed in early follicular and mid-luteal phases of the menstrual cycle as well as testosterone, androstenedione, progesterone, dehydroepiandrosterone (DHEA), DHEA sulfate, sex hormone–binding globulin (SHBG), and anti-Müllerian hormone in luteal or untimed samples. We used multivariable linear regression to quantify the association of %MD with quartiles of each hormone, adjusting for age, body mass index, and breast cancer risk factors. Women in the highest quartile of follicular estradiol levels had significantly greater %MD compared to those in the lowest quartile [difference, 6.7 percentage points; 95% confidence interval (CI) 2.2, 11.3; p-trend < 0.001]. Similar associations were observed for follicular free estradiol but not luteal-phase estradiol. Also, women in the top (vs. bottom) quartile of free testosterone had significantly lower %MD (difference, − 4.7; 95% CI − 8.7, − 0.8; p-trend = 0.04). Higher SHBG was significantly associated with higher percent MD (difference, 4.8; 95% CI 1.1, 8.6; p-trend = 0.002). Percent MD was not strongly associated with other measured hormones. Results were similar in analyses that excluded women with anovulatory cycles. Our findings suggest that follicular estradiol and SHBG may play an important role in premenopausal percent MD.
Electronic supplementary material
The online version of this article (10.1007/s12672-017-0321-6) contains supplementary material, which is available to authorized users.
Keywords: Mammographic Density (MD), Sex Hormone-binding Globulin (SHBG), Follicular Estradiol, Untimed Samples, Higher SHBG
Introduction
Prior research suggests that endogenous sex steroids are associated with breast cancer risk in premenopausal women [1–3], while mammographic density (MD), a strong and independent risk factor for breast cancer [4], may reflect cumulative exposure to estrogens [5]. A few studies, but not all, have reported positive associations between estrogens and MD, primarily among postmenopausal women [6–12], suggesting at least one possible biological mechanism of action through which hormones may influence breast cancer risk. However, fewer studies have evaluated the association between endogenous hormones and MD among premenopausal women [12–18]. Results of these studies have been inconsistent, in part because of cyclical variations in estrogen levels across the menstrual cycle. Similarly, data to evaluate the association with circulating androgens are also sparse [13, 15, 16, 19]. To date, only a single study [20] assessed possible associations of serum anti-Müllerian hormone (AMH), a marker of ovarian function which has been found in three prospective studies to be positively associated with breast cancer risk [21–23], and breast density in younger women (n = 172). Findings from that study suggested no association of AMH with absolute or percent breast dense volume; however, an inverse association was observed for absolute non-dense breast volume.
Understanding how endogenous hormone levels influence MD may inform breast cancer etiology. Therefore, to address the gaps in knowledge about the relation of circulating hormones to MD, we evaluated associations within the Nurses’ Health Study II (NHSII).
Methods
Study Population
The NHSII is an ongoing prospective cohort study of 116,429 women who were ages 25 to 42 at baseline in 1989. Self-administered questionnaires were administered at baseline to collect information on diseases and risk factors such as weight, family history of breast cancer, age at menarche, parity, alcohol consumption, and use of oral contraceptives. Biennial questionnaires update information on most risk factors. Blood samples were collected in 1996–1999 from 29,611 women in the NHSII, including 18,521 premenopausal women who provided samples timed during the early follicular and mid-luteal phases of their menstrual cycles [24]. Samples have been stored in liquid nitrogen freezers (<− 130 °C) since collection. Within the subcohort of women who provided blood samples, a nested case–control study of breast cancer was established to investigate a wide range of biomarkers as potential predictors of breast cancer risk, as described previously [24–26]. Briefly, we identified new diagnoses of breast cancer through biennial questionnaires and regular searches of the National Death Index and confirmed diagnoses through medical record review. Two controls with no prior history of breast or other cancer were matched to each case by race/ethnicity (African-American, Asian, Hispanic, Caucasian, other), age (±2 years), menopausal status, month/year of blood collection, time of day of blood draw (±2 h), fasting status, and luteal day for timed samples (date of next period minus date of luteal blood draw, ±1 day) [24].
Film-screen mammograms were collected from women included in the nested case–control study. Screening mammograms were obtained as close as possible to the time of blood collection (median time from blood to mammogram, 7 months), and we successfully obtained mammograms from approximately 80% of eligible women (i.e., current participants in the nested case–control study who reported having received mammography). Women from whom we did and did not receive mammograms were similar with regard to breast cancer risk factors, including body mass index (BMI), parity, and family history of breast cancer [11]. We conducted cross-sectional analyses among controls from this nested case–control study. We restricted all analyses to women who were premenopausal at the time of both mammography and blood collection. Distributions of hormone concentrations were similar for controls with mammograms versus all controls (data not shown). The final analytic sample consisted of 634 women. This study was approved by the institutional review board of Brigham and Women’s Hospital. Informed consent was implied by receipt of completed questionnaires and blood samples.
Mammographic Density (MD) Measurements
Assessment of MD has been described previously [11]. Briefly, we measured absolute and percent MD from the craniocaudal views of both breasts using Cumulus software [27]. All images were read by a single reader; within NHSII, mammograms were read in two batches approximately 3 years apart. The within-person intraclass correlation coefficients were ≥ 0.90 [28]; however, there was evidence of batch-to-batch variability in density measurements so MD measurements were adjusted for batch effects using a correction technique described by Rosner et al. [11, 29].
We used the average percent density of both breasts for our main analyses as this is more strongly related to breast cancer risk than absolute density phenotypes [8, 30]. However, recent evidence suggests that absolute dense and non-dense area may be independently associated with breast cancer risk [28, 31–33], so we also examined these as separate outcomes in secondary analyses.
Laboratory Analyses
The details of laboratory assay methods used to quantify plasma concentrations of estrogens, androgens, progesterone, sex hormone–binding globulin (SHBG), and AMH have been described previously [23, 34, 35]. Luteal and follicular samples were assayed for estrone, estradiol, and estrone sulfate. Testosterone and androstenedione concentrations were assayed in luteal and/or follicular samples, as well as untimed samples. Dehydroepiandrosterone (DHEA), DHEA sulfate (DHEAS), SHBG, and AMH were measured in luteal and untimed samples, and progesterone was measured in luteal samples. Assays were performed in different batches at different laboratories [36]. Hormones were assayed by radioimmunoassay or liquid chromatography–tandem mass spectrometry (estrogens, testosterone, androestenedione) and chemiluminescence immunoassay (progesterone, DHEA, DHEAS, SHBG). Samples were assayed for AMH, in a single batch, by the picoAMH ELISA assay at Ansh Labs [23]. We included 10% blinded replicates in each batch to assess laboratory precision. Except for a single batch of progesterone (17%), within-batch coefficients of variation were between 2 and 15% for all analytes. Free estradiol and free testosterone were calculated using the formula described by Södergard et al. [37].
Statistical Analyses
Because sex steroid hormone levels vary throughout the menstrual cycle, we evaluated estrone and estradiol in the follicular and luteal phases separately, and progesterone in the luteal phase. We used the average of the follicular and luteal blood sample values, when available, for testosterone, free testosterone, and androstenedione as concentrations did not vary substantially by menstrual phase [38]. We adjusted for between-batch differences in hormone distributions (for all hormones except AMH) using an average batch recalibration approach [3, 29]. Sample sizes ranged from 230 (for analyses of AMH) to 631 (for SHBG).
In cross-sectional analyses, we fit multivariable linear regression models to quantify the association of %MD with quartiles of each hormone, adjusting for age and age-squared, body mass index (BMI) at blood collection (continuous, kg/m2), age at menarche (< 12, 12, 13, ≥ 14 years), parity and age at first birth (nulliparous, 1–2 children and < 25 years, 1–2 children and 25–29 years, 1–2 children and ≥ 30 years, 3+ children and < 25 years, 3+ children and ≥ 25 years, missing), family history of breast cancer at blood draw (yes, no), alcohol intake at blood draw (0, 0.1–4.9, 5–14.9, 15+ g/day, missing), and luteal day (3–7 days, 8–28 days, missing/untimed). Risk factor information was based on questionnaires completed at the time of blood collection (i.e., weight) or from biennial questionnaires completed close to the time of blood collection. A missing indicator category was used to account for missing values in categorical covariates. Because of strong inverse correlations between BMI and percent MD [39–41], results from models adjusted for age only are not shown. We present models adjusted for age, age-squared, and BMI alone, plus full multivariable models. In secondary analyses incorporating absolute measures of dense and non-dense breast area, we applied a square-root transformation to improve normality of these outcomes. Models for absolute dense area were adjusted for absolute non-dense area and vice versa. Generalized estimating equations were used to take into account the correlation between matched controls. Statistical tests for trend were from a Wald test using the median of each quartile as a continuous variable. We examined the possibly non-linear relation between hormones and percent MD non-parametrically with restricted cubic splines [42]. Tests for non-linearity used the likelihood ratio test, comparing the model with only the linear term to the model with the linear and the cubic spline terms. In sensitivity analyses for estrogens, we excluded women whose samples were collected in an anovulatory cycle [defined by luteal progesterone <400 ng/dL (n = 67) or missing (n = 4)]. We also conducted analyses stratified by BMI (< 25 vs. ≥ 25 kg/m2) and formally tested for interaction by evaluating the Wald test for the cross-product term of dichotomous BMI and continuous hormone in multivariable models.
Analyses were conducted with SAS version 9.3 for UNIX (SAS Institute, Cary, NC). All p values were based on two-sided tests and were considered statistically significant if < 0.05.
Results
On average, women were 42.8 years of age at blood draw and 44.1 years at mammogram, with average percent MD of 43.0%. Other characteristics of the study population are shown in Table 1.
Table 1.
Selected characteristics of the study population at the time of blood collection (n = 634)a
| Age at blood collection, years | 42.8 |
| Age at mammogram, years | 44.1 |
| Average mammographic density, % | 43.0 |
| Body mass index, kg/m2 | 25.3 |
| Family history of breast cancer,% | 9.5 |
| Ever used oral contraceptives, % | 84.2 |
| Age at menarche, % | |
| < 12 years | 24.1 |
| 12 years | 28.5 |
| 13 years | 29.3 |
| 14+ years | 18.1 |
| Parity and age at first birth, % | |
| Nulliparous | 18.0 |
| 1–2 kids, < 25 years | 11.8 |
| 1–2 kids, 25–29 years | 21.9 |
| 1–2 kids, 30+ years | 16.4 |
| 3+ kids, < 25 years | 14.5 |
| 3+ kids, 25+ years | 16.4 |
| Alcohol consumption, % | |
| None | 36.5 |
| 0.1–4.9 g/day | 38.5 |
| 5–14.9 g/day | 16.3 |
| 15+ g/day | 4.6 |
| Missing | 4.1 |
| Luteal day, % | |
| 3–7 days | 42.1 |
| 8–28 days | 37.4 |
| Missing/untimed | 20.5 |
aValues are means or %
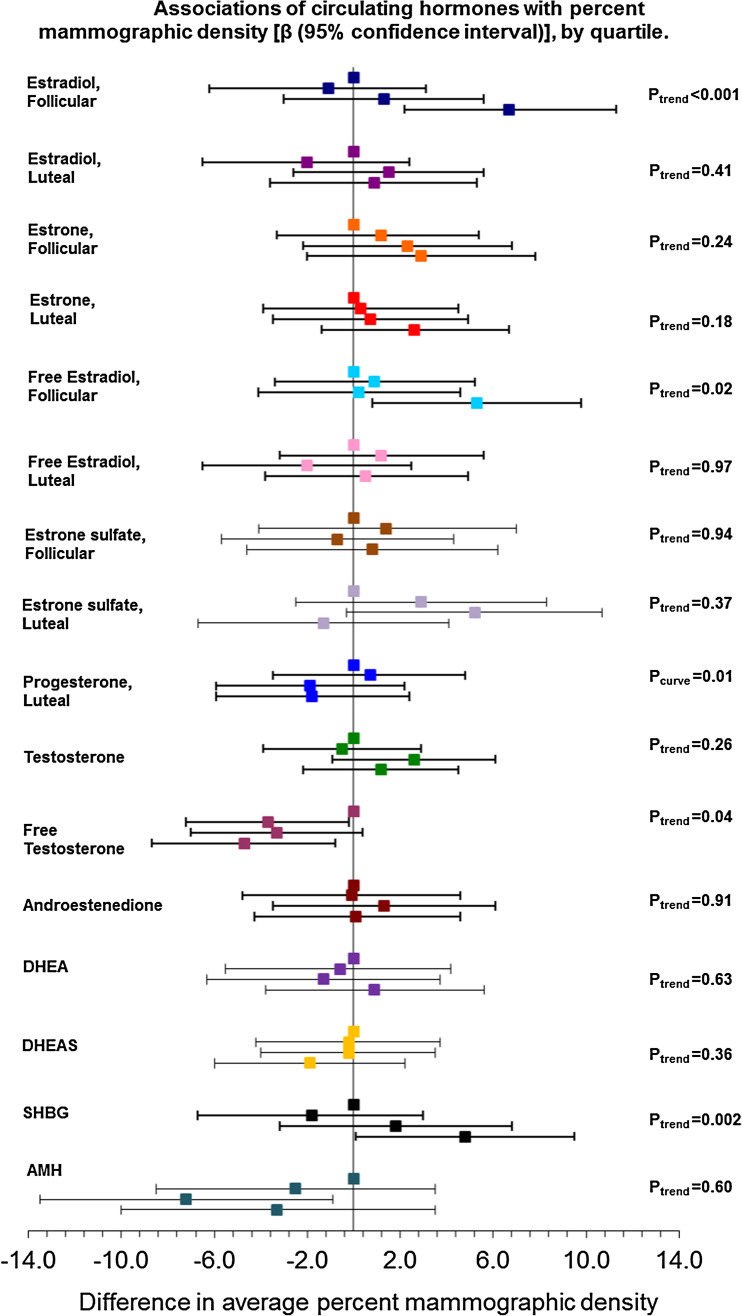
In general, after adjustment for age and BMI, we did not observe strong evidence of confounding of associations of plasma hormones and %MD by the other breast cancer risk factors (Table 2 and Fig. 1). In full multivariable-adjusted models (Table 2, Model 2), %MD was significantly higher among women in the highest quartile of follicular estradiol levels compared to those in the lowest quartile (difference, 6.7 percentage points; 95% CI 2.2, 11.3; p-trend < 0.001). Similar associations were observed for follicular free estradiol (correlation with total estradiol = 0.84) but not luteal-phase estradiol (Table 2). These associations were similar in analyses restricted to ovulatory women (data not shown). In addition, women in the top (vs. bottom) quartile of free testosterone had significantly lower %MD (difference, − 4.7; 95% CI − 8.7, − 0.8; p-trend = 0.04). Higher SHBG was significantly associated with higher percent MD (p-trend < 0.01) (Table 2); the association was somewhat attenuated when mutually adjusted for follicular total estradiol (p-trend = 0.11). Percent MD was not associated with estrone, estrone sulfate, testosterone, androstenedione, DHEA, DHEAS, or AMH (Fig. 1 and Table 2). For most hormones, there was no evidence of non-linearity in associations of hormones with percent MD; the single exception was progesterone, for which there was significant evidence of a non-linear association (p ≤ 0.01).
Table 2.
Difference in average percent mammographic density [β (95% confidence interval)] by quartile of plasma hormone exposure (maximum n = 631)
| Plasma hormone | Quartiles | p-trend* | ||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | |||
| Estradiol | ||||||
| Follicular (n = 389) | Median, pg/mL | 21.2 | 37.7 | 50.5 | 82.6 | |
| Model 1 | Ref | − 1.6 (− 6.2, 3.1) | 0.5 (− 3.9, 4.8) | 6.3 (1.6, 11.0) | 0.002 | |
| Model 2 | Ref | − 1.1 (− 5.7, 3.5) | 1.3 (− 3.0, 5.6) | 6.7 (2.2, 11.3) | < 0.001 | |
| Luteal (n = 396) | Median, pg/mL | 73.1 | 109.6 | 145.1 | 203.7 | |
| Model 1 | Ref | − 1.2 (− 5.9, 3.5) | 1.5 (− 2.8, 5.8) | 0.3 (− 4.1, 4.6) | 0.68 | |
| Model 2 | Ref | − 2.0 (− 6.5, 2.4) | 1.5 (− 2.6, 5.6) | 0.9 (− 3.6, 5.3) | 0.41 | |
| Estrone | ||||||
| Follicular (n = 395) | Median, pg/mL | 26.1 | 35.5 | 43.9 | 62.1 | |
| Model 1 | Ref | 1.0 (− 3.3, 5.4) | 0.8 (− 3.6, 5.2) | 1.5 (− 3.3, 6.3) | 0.59 | |
| Model 2 | Ref | 1.2 (− 3.0, 5.4) | 2.3 (− 2.2, 6.8) | 2.9 (− 2.0, 7.8) | 0.24 | |
| Luteal (n = 429) | Median, pg/mL | 51.5 | 73.1 | 89.6 | 122.5 | |
| Model 1 | Ref | 0.8 (− 3.3, 5.0) | − 0.1 (− 4.3, 4.1) | 1.5 (− 2.5, 5.5) | 0.53 | |
| Model 2 | Ref | 0.3 (− 3.9, 4.5) | 0.7 (− 3.5, 4.9) | 2.6 (− 1.4, 6.7) | 0.18 | |
| Free estradiol | ||||||
| Follicular (n = 382) | Median, pg/mL | 0.3 | 0.5 | 0.7 | 1.0 | |
| Model 1 | Ref | − 0.5 (− 4.9, 3.9) | − 0.6 (− 5.1, 3.9) | 3.6 (− 1.1, 8.3) | 0.11 | |
| Model 2 | Ref | 0.9 (− 3.4, 5.2) | 0.2 (− 4.1, 4.6) | 5.3 (0.8, 9.8) | 0.02 | |
| Luteal (n = 391) | Median, pg/mL | 0.9 | 1.4 | 1.8 | 2.6 | |
| Model 1 | Ref | 1.0 (− 3.6, 5.6) | − 2.2 (− 6.6, 2.3) | − 0.2 (− 4.4, 4.1) | 0.69 | |
| Model 2 | Ref | 1.2 (− 3.2, 5.6) | − 2.0 (− 6.5, 2.5) | 0.5 (− 3.8, 4.9) | 0.97 | |
| Estrone sulfate | ||||||
| Follicular (n = 269) | Median, pg/mL | 315.4 | 532.9 | 778.0 | 1295.6 | |
| Model 1 | Ref | 0.1 (− 5.5, 5.6) | − 2.6 (− 7.9, 2.8) | − 0.6 (− 6.3, 5.1) | 0.73 | |
| Model 2 | Ref | 1.4 (− 4.1, 7.0) | − 0.7 (− 5.7, 4.3) | 0.8 (− 4.6, 6.2) | 0.94 | |
| Luteal (n = 267) | Median, pg/mL | 649.3 | 1227.2 | 1869.4 | 3427.5 | |
| Model 1 | Ref | 1.1 (− 4.1, 6.4) | 4.7 (− 0.5, 9.9) | − 1.6 (− 6.6, 3.3) | 0.44 | |
| Model 2 | Ref | 2.9 (− 2.5, 8.3) | 5.2 (− 0.3, 10.7) | − 1.3 (− 6.7, 4.1) | 0.37 | |
| Progesterone | ||||||
| Luteal (n = 531) | Median, ng/dL | 324.5 | 991.0 | 1440.8 | 2147.2 | |
| Model 1 | Ref | − 0.3 (− 4.2, 3.5) | − 3.3 (− 7.2, 0.5) | − 3.1 (− 6.9, 0.7) | ** | |
| Model 2 | Ref | 0.7 (− 3.5, 4.8) | − 1.9 (− 5.9, 2.2) | − 1.8 (− 5.9, 2.4) | ** | |
| Testosterone | ||||||
| Average + untimed (n = 630) | Median, ng/dL | 15.1 | 20.8 | 26.4 | 35.0 | |
| Model 1 | Ref | − 1.2 (− 4.7, 2.4) | 2.3 (− 1.3, 5.8) | 0.4 (− 3.0, 3.7) | 0.46 | |
| Model 2 | Ref | − 0.5 (− 3.9, 2.9) | 2.6 (− 0.9, 6.1) | 1.2 (− 2.2, 4.5) | 0.26 | |
| Free testosterone | ||||||
| Average + untimed (n = 624) | Median, ng/dL | 0.11 | 0.16 | 0.21 | 0.32 | |
| Model 1 | Ref | − 3.9 (− 7.6, − 0.2) | − 3.3 (− 7.0, 0.5) | − 5.1 (− 9.1, − 1.1) | 0.03 | |
| Model 2 | Ref | − 3.7 (− 7.2, − 0.2) | − 3.3 (− 7.0, 0.4) | − 4.7 (− 8.7, − 0.8) | 0.04 | |
| Androstenedione | ||||||
| Average + untimed (n = 352) | Median, ng/dL | 61.0 | 85.8 | 106.3 | 147.6 | |
| Model 1 | Ref | 0.4 (− 4.3, 5.1) | 1.4 (− 3.5, 6.3) | − 0.4 (− 5.1, 4.3) | 0.88 | |
| Model 2 | Ref | − 0.1 (− 4.8, 4.6) | 1.3 (− 3.5, 6.1) | 0.1 (− 4.3, 4.6) | 0.91 | |
| DHEA | ||||||
| Luteal + untimed (n = 349) | Median, ng/dL | 386.4 | 544.9 | 712.7 | 1061.6 | |
| Model 1 | Ref | − 0.2 (− 5.0, 4.6) | − 0.3 (− 5.4, 4.8) | 1.7 (− 3.2, 6.7) | 0.44 | |
| Model 2 | Ref | − 0.6 (− 5.5, 4.2) | − 1.3 (− 6.3, 3.7) | 0.9 (− 3.8, 5.6) | 0.63 | |
| DHEAS | ||||||
| Luteal + untimed (n = 508) | Median, μg/dL | 32.6 | 55.8 | 80.0 | 116.9 | |
| Model 1 | Ref | 0.0 (− 4.0, 4.0) | 0.1 (− 3.7, 4.0) | − 0.9 (− 4.9, 3.0) | 0.64 | |
| Model 2 | Ref | − 0.2 (− 4.2, 3.7) | − 0.2 (− 4.0, 3.5) | − 1.9 (− 6.0, 2.2) | 0.36 | |
| SHBG | ||||||
| Luteal + untimed (n = 631) | Median, nmol/L | 35.5 | 54.5 | 74.9 | 106.2 | |
| Model 1 | Ref | − 2.5 (− 6.2, 1.1) | 1.7 (− 2.2, 5.5) | 4.6 (0.8, 8.5) | 0.002 | |
| Model 2 | Ref | − 1.8 (− 5.3, 1.7) | 1.8 (− 2.1, 5.6) | 4.8 (1.1, 8.6) | 0.002 | |
| AMH | ||||||
| Luteal + untimed (n = 230) | Median, pg/mL | 131.0 | 539.9 | 1146.4 | 2806.3 | |
| Model 1 | Ref | − 1.6 (− 7.7, 4.5) | − 6.9 (− 13.1, − 0.6) | − 1.7 (− 8.6, 5.2) | 0.93 | |
| Model 2 | Ref | − 2.5 (− 8.5, 3.5) | − 7.2 (− 13.5, − 0.9) | − 3.3 (− 10.0, 3.5) | 0.60 | |
DHEA dehydroepiandrosterone, DHEAS dehydroepiandrosterone sulfate, SHBG sex hormone–binding globulin, AMH anti-Müllerian hormone
Model 1: adjusted for age, age2 at blood draw, and BMI at blood draw
Model 2: adjusted for age and age2 at blood draw, BMI at blood draw (continuous, kg/m2), age at menarche (< 12, 12, 13, ≥ 14), parity and age at first birth (nulliparous, 1–2 children and < 25 years, 1–2 children and 25–29 years, 1–2 children and ≥ 30 years, 3+ children and < 25 years, 3+ children and ≥ 25 years + missing), family history of breast cancer at blood draw (yes, no), alcohol intake at blood draw (0 g/day, 0.1–4.9 g/day, 5–14.9 g/day, 15 + g/day, missing), luteal day (3–7 days, 8–28 days, missing/untimed)
*Trend test with median value of the quartile
**Significant evidence of non-linearity (p ≤ 0.01); p values for overall significance of curve were < 0.01 for Model 1 and 0.01 for Model 2
Fig. 1.
Difference in average percent mammographic density by quartile of plasma hormone exposure (quartiles are 1 to 4 from top to bottom). Beta estimates and 95% confidence intervals are the same as shown in Table 2 (Model 2) and are based on multivariable models adjusted for age and age2 at blood draw, BMI at blood draw (continuous, kg/m2), age at menarche (< 12, 12, 13, ≥ 14), parity and age at first birth (nulliparous, 1–2 children and < 25 years, 1–2 children and 25–29 years, 1–2 children and ≥ 30 years, 3+ children and < 25 years, 3+ children and ≥ 25 years + missing), family history of breast cancer at blood draw (yes, no), alcohol intake at blood draw (0 g/day, 0.1–4.9 g/day, 5–14.9 g/day, 15 + g/day, missing), luteal day (3–7 days, 8–28 days, missing/untimed). Ptrend is the test for trend based on the median value of each quartile. DHEA, dehydroepiandrosterone; DHEAS, dehydroepiandrosterone sulfate; SHBG, sex hormone–binding globulin; AMH, anti-Müllerian hormone
In analyses stratified by BMI, we observed that associations were generally stronger in overweight and obese women with BMI ≥ 25 kg/m2. For example, among leaner women, a significant positive association was observed for follicular estradiol (difference between extreme quartiles, 5.0; 95% CI − 0.4, 10.4; p-trend = 0.04). Although there was no significant interaction by BMI (p-interaction = 0.35), the magnitude of the association was stronger among heavier women: percent MD for those in the top quartile of follicular estradiol was 13.9 percentage points higher than those in the bottom quartile (95% CI 6.6, 21.2; p-trend < 0.0001) (Table 3). The associations observed with free testosterone (inverse) and SHBG (positive) overall seemed to be driven mainly by associations in overweight and obese women, among whom significant inverse associations were also observed with androstenedione and DHEAS. In contrast, the androgens did not appear to be strongly associated with percent MD among leaner women; however, a suggestive inverse association for AMH was observed in this group. Significant non-linear associations were observed for progesterone in both strata of BMI and SHBG in heavier women (Table 3).
Table 3.
Difference in average percent mammographic density [β (95% confidence interval)] by quartile of plasma hormone exposure, stratified by BMI
| Plasma hormone | BMI (kg/m2) | N | Quartiles | p-trend* | p-int^ | |||
|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | |||||
| Estradiol | ||||||||
| Follicular | ≤ 25 | 244 | Ref | 0.8 (− 5.0, 6.7) | 0.7 (− 5.1, 6.5) | 5.0 (− 0.4, 10.4) | 0.04 | 0.35 |
| > 25 | 144 | Ref | − 1.3 (− 8.1, 5.6) | 4.0 (− 2.0, 10.1) | 13.9 (6.6, 21.2) | < 0.0001 | ||
| Luteal | ≤ 25 | 251 | Ref | 2.3 (− 3.7, 8.2) | 6.0 (0.9, 11.1) | 2.5 (− 3.2, 8.2) | 0.58 | 0.05 |
| > 25 | 144 | Ref | − 5.1 (− 11.2, 1.0) | 0.4 (− 6.3, 7.2) | 5.5 (− 2.3, 13.3) | 0.12 | ||
| Estrone | ||||||||
| Follicular | ≤ 25 | 247 | Ref | 0.7 (− 3.9, 5.4) | − 2.7 (− 8.1, 2.6) | 2.2 (− 2.9, 7.4) | 0.50 | 0.18 |
| > 25 | 147 | Ref | 2.0 (− 4.7, 8.6) | 8.7 (1.8, 15.7) | 4.9 (− 3.7, 13.4) | 0.24 | ||
| Luteal | ≤ 25 | 269 | Ref | − 0.3 (− 5.5, 4.9) | − 0.1 (− 5.1, 4.8) | 0.3 (− 4.6, 5.1) | 0.88 | 0.22 |
| > 25 | 159 | Ref | − 18.2 (− 25.6, − 10.7) | − 18.1 (− 31.2, − 5.0) | − 11.7 (− 24.3, 0.9) | 0.40 | ||
| Free estradiol | ||||||||
| Follicular | ≤ 25 | 238 | Ref | 2.7 (− 2.5, 7.8) | 2.4 (− 3.3, 8.0) | 4.6 (− 0.7, 9.9) | 0.11 | 0.26 |
| > 25 | 143 | Ref | − 1.2 (− 7.9, 5.5) | − 5.0 (− 11.9, 1.8) | 8.2 (1.2, 15.2) | 0.02 | ||
| Luteal | ≤ 25 | 247 | Ref | 0.5 (− 5.0, 5.9) | 1.1 (− 4.6, 6.8) | 0.7 (− 4.3, 5.7) | 0.85 | 0.09 |
| > 25 | 143 | Ref | 2.7 (− 4.5, 9.8) | − 1.3 (− 8.1, 5.5) | 4.9 (− 2.0, 11.8) | 0.26 | ||
| Estrone sulfate | ||||||||
| Follicular | ≤ 25 | 167 | Ref | − 2.1 (− 8.4, 4.1) | 0.8 (− 4.7, 6.3) | 0.3 (− 5.4, 5.9) | 0.69 | 0.17 |
| > 25 | 102 | Ref | 6.8 (− 1.7, 15.4) | 2.6 (− 4.6, 9.8) | 4.8 (− 4.2, 13.8) | 0.57 | ||
| Luteal | ≤ 25 | 173 | Ref | 3.0 (− 2.6, 8.7) | 4.0 (− 1.5, 9.5) | 0.0 (− 5.9, 5.8) | 0.63 | 0.10 |
| > 25 | 94 | Ref | 2.2 (− 6.9, 11.3) | 9.0 (− 1.0, 19.0) | − 5.6 (− 15.1, 3.9) | 0.19 | ||
| Progesterone | ||||||||
| Luteal | ≤ 25 | 331 | Ref | − 1.1 (− 5.9, 3.7) | − 3.7 (− 8.1, 0.8) | − 4.3 (− 8.9, 0.2) | ** | 0.39 |
| > 25 | 199 | Ref | − 3.8 (− 19.5, 11.8) | − 11.7 (− 28.0, 4.5) | − 2.9 (− 16.5, 10.6) | ** | ||
| Testosterone | ||||||||
| Average + untimed | ≤ 25 | 386 | Ref | 0.4 (− 3.7, 4.5) | 3.1 (− 1.2, 7.4) | 2.1 (− 2.0, 6.2) | 0.20 | < 0.01 |
| > 25 | 243 | Ref | − 4.2 (− 9.9, 1.5) | 0.0 (− 6.2, 6.2) | − 2.5 (− 8.1, 3.1) | 0.78 | ||
| Free testosterone | ||||||||
| Average + untimed | ≤ 25 | 380 | Ref | − 0.1 (− 3.8, 3.7) | 0.9 (− 3.4, 5.1) | − 0.5 (− 5.1, 4.1) | 0.92 | < 0.01 |
| > 25 | 243 | Ref | − 8.8 (− 16.1, − 1.6) | − 8.2 (− 15.4, − 0.9) | − 10.5 (− 17.1, − 3.9) | 0.01 | ||
| Androstenedione | ||||||||
| Average + untimed | ≤ 25 | 219 | Ref | 4.3 (− 0.8, 9.5) | 3.2 (− 2.2, 8.7) | 3.2 (− 1.8, 8.2) | 0.43 | 0.03 |
| > 25 | 133 | Ref | − 8.6 (− 15.7, − 1.5) | − 1.9 (− 9.8, 5.9) | − 6.9 (− 15.3, 1.6) | < 0.0001 | ||
| DHEA | ||||||||
| Luteal + untimed | ≤ 25 | 216 | Ref | − 0.8 (− 6.4, 4.7) | 0.4 (− 4.9, 5.7) | 2.6 (− 2.6, 7.8) | 0.19 | 0.10 |
| > 25 | 133 | Ref | − 3.4 (− 10.7, 4.0) | − 0.9 (− 10.1, 8.3) | − 0.3 (− 8.0, 7.3) | ** | ||
| DHEAS | ||||||||
| Luteal + untimed | ≤ 25 | 314 | Ref | − 0.8 (− 5.6, 4.1) | 0.8 (− 3.6, 5.2) | 0.4 (− 4.1, 4.9) | 0.69 | 0.12 |
| > 25 | 193 | Ref | 1.4 (− 5.5, 8.3) | 1.4 (− 4.8, 7.6) | − 5.4 (− 11.7, 0.8) | 0.05 | ||
| SHBG | ||||||||
| Luteal + untimed | ≤ 25 | 386 | Ref | − 2.9 (− 8.1, 2.3) | − 1.8 (− 6.7, 3.1) | − 0.4 (− 5.1, 4.4) | 0.53 | 0.16 |
| > 25 | 244 | Ref | − 1.5 (− 5.8, 2.8) | 5.8 (0.0, 11.6) | 7.7 (− 0.4, 15.9) | 0.02 | ||
| AMH | ||||||||
| Luteal + untimed | ≤ 25 | 142 | Ref | − 3.2 (− 9.4, 2.9) | − 8.0 (− 15.4, − 0.6) | − 5.4 (− 13.1, 2.4) | 0.46 | 0.55 |
| > 25 | 88 | Ref | − 0.6 (− 12.7, 11.4) | − 9.6 (− 19.9, 0.7) | − 1.5 (− 13.2, 10.2) | 0.68 | ||
DHEA dehydroepiandrosterone, DHEAS dehydroepiandrosterone sulfate, SHBG sex hormone–binding globulin, AMH anti-Müllerian hormone
Model is adjusted for age and age2 at blood draw, BMI at blood draw (continuous, kg/m2), age at menarche (< 12, 12, 13, ≥ 14), parity and age at first birth (nulliparous, 1–2 children and <25 years, 1–2 children and 25–29 years, 1–2 children and ≥ 30 years, 3+ children and < 25 years, 3+ children and ≥ 25 years + missing), family history of breast cancer at blood draw (yes, no), alcohol intake at blood draw (0 g/day, 0.1–4.9 g/day, 5–14.9 g/day, 15 + g/day, missing), luteal day (3–7 days, 8–28 days, missing/untimed)
Refer to Table 2 for quartile medians
*Trend test with median value of the quartile
**Significant evidence of non-linearity (p ≤ 0.03); p values for overall significance of curve were < 0.01 for progesterone in stratum of BMI ≤ 25, 0.04 for progesterone in stratum of BMI > 25, and 0.01 for DHEA in stratum of BMI > 25
^Test for interaction with BMI
Similar to our main analyses for percent MD, we observed positive associations between follicular estradiol and free estradiol with absolute dense breast area (p-trend < 0.01) after adjusting for age, BMI, absolute non-dense area, and other breast cancer risk factors (Supplementary Table 1). The positive association between SHBG and percent MD was driven by a strong inverse association of SHBG with absolute non-dense breast area (p-trend < 0.001) (Supplementary Table 2), whereas there was no apparent association of SHBG with absolute dense breast area (Supplementary Table 1). No clear trends were noted for the other plasma hormones evaluated and absolute non-dense area. However, there was a borderline inverse association between AMH and absolute dense breast area (p-trend = 0.05) (Supplementary Table 1).
Discussion
In summary, we observed a significant positive association between follicular-phase estradiol and percent MD in premenopausal women. We previously reported that follicular estradiol was associated with invasive and ER+/PR+ premenopausal breast cancer [3]. Other studies have also reported positive associations of circulating estrogens with breast cancer risk in premenopausal women [1]. Our current results support the hypothesis that the association could be mediated, at least in part, through high MD. We also observed an inverse association with free testosterone, which was likely driven by the inverse association of SHBG with absolute non-dense breast area resulting in a positive association of SHBG with percent MD that was mainly apparent among overweight and obese women.
In a subset of this population (n = 352), we previously reported no associations between urinary estrogen metabolites and MD [11]. However, the current results are not inconsistent with our previous analyses since urinary estrogen metabolites were measured in urine samples and during the luteal phase of the menstrual cycle, whereas we observed that plasma levels of estradiol measured in the follicular phase (but not the luteal phase) were positively associated with percent MD. Few prior studies have evaluated phase-specific circulating sex hormones and MD in premenopausal women with mixed results. Similar to our findings, Yong et al. reported a positive association of similar magnitude between follicular phase estradiol and MD among 192 premenopausal women aged 40–45 [15]. However, results from three other studies that measured circulating hormone levels (n ≤ 225) were null [12, 16, 43]. In one of these studies, however, both follicular-phase and overall average salivary concentrations of 17β-estradiol (measured daily throughout the menstrual cycle) were positively associated with percent MD in premenopausal women ages 25–35 (n = 202) [16].
Our findings for SHBG (positive) and free testosterone (inverse) are also consistent with the findings of Yong et al. [15] and with a recent analysis of 225 younger women aged 15–30 years in which SHBG measured in both the follicular and luteal phases was found to be positively associated with percent breast water measured using MRI (as a proxy for breast density) [43]. In the latter study, an inverse association for free testosterone was also noted. Walker et al. also reported a suggestive positive association between SHBG and percent MD in 494 premenopausal women [13]. In contrast, among 180 women aged 25–29, Jung et al. found a positive association between testosterone and percent breast density, but no association with SHBG or non-SHBG bound testosterone [14]. The role of SHBG in the body is one of transport and it binds both androgens and estrogens; however, SHBG has a higher affinity for testosterone [44] than estradiol and SHBG concentrations are negatively correlated with testosterone levels but positively correlated with estradiol levels in premenopausal women [38, 45]. In these data, SHBG is negatively correlated with free testosterone (r = − 0.51) and positively correlated with estradiol (r = 0.52). Therefore, it is possible that the positive association we observed between SHBG and percent MD in our analyses largely reflect the positive association of estradiol and free estradiol in this study population; indeed, adjustment for estradiol somewhat attenuated observed associations of SHBG with percent MD. However, Linton et al. did not observe associations of estradiol in either phase of the menstrual cycle with percent breast water from MRI [43]. Although we adjusted for BMI in our analyses, it is also possible that our findings for SHBG reflect residual confounding by adiposity, given that the association was stronger and non-linear among overweight/obese women and the strong inverse association of SHBG with absolute non-dense breast area.
We also found that while follicular estradiol was positively associated with percent MD in both lean and heavier women, the association was stronger among overweight and obese women. While estrogens may have a direct influence on breast tissue composition [5], adiposity—and associated metabolic processes such as the insulin-like growth factor (IGF)-1 pathway—could modify the biological effects of sex hormones. Interactions between estrogen and IGF-1 on breast cancer development have been described [46, 47], and recently, Frydenberg et al. [48] reported stronger associations of salivary 17β-estradiol with percent MD among premenopausal women who also had high levels of IGF-1 or growth hormone (n = 99). However, IGF-1 and growth hormone are inversely associated with BMI [49] and, in an earlier analysis, we found no association of IGF-1 and MD in this cohort [50]. These interactions warrant further investigation in larger and prospective studies.
We found no linear association overall with progesterone measured during the luteal phase; however, we detected significant evidence of non-linearity in the association of progesterone with percent MD. Variable results in the literature [17, 19] may be due in part to measurement challenges: the ICC for luteal-phase progesterone in the NHSII was only 0.29 [38], and these results should be interpreted with caution.
To our knowledge, only one prior study evaluated the relation between AMH and breast density. Among 172 women aged 25–29, AMH levels were not associated with percent breast density; however, women with higher AMH concentrations had significantly lower non-dense breast volume [20]. In contrast, we noted a suggestive inverse association of AMH with absolute dense breast area, but no association with absolute non-dense breast area. The discrepancy in results between these two studies could be due to differences in the ages of the study populations or may be due to chance.
There are some important limitations of our analysis. First, because of the cross-sectional study design, in which blood samples and mammograms were obtained close in time to each other, temporality cannot be inferred. Second, a single blood sample (i.e., follicular or luteal) may not accurately represent long-term average hormone levels or the relevant etiologic period. In a reproducibility study within the NHS II, with the exception of progesterone, most hormones measured at specific points in the menstrual phase were fairly stable over 1–3 years (e.g., estradiol ICC = 0.45, testosterone ICC = 0.69, SHBG ICC = 0.83) [3, 24, 38]. Mammograms were not performed at the same time as blood draw and we lacked information of timing of mammography with respect to the menstrual cycle; however, previous studies suggest only negligible differences in density measures at different points in the menstrual cycle [51, 52]. Finally, while results were similar in analyses that excluded women with an anovulatory cycle at blood draw, a single anovulatory cycle may not be representative of usual cycles and we were unable to characterize women according to history of regular ovulatory cycles.
Despite these limitations, there are important strengths as well. Our study is the largest, to our knowledge, to evaluate associations between sex steroid hormones by menstrual timing and premenopausal MD. Other strengths of this study include quantitative assessments of percent and absolute MD from screening mammograms with high intra-reader reliability, use of state-of-the-art hormone assays, and detailed information on potential confounders, including predictors of MD and established breast cancer risk factors.
Our findings suggest that follicular estradiol and possibly SHBG may play important roles in MD among premenopausal women. Further research is warranted to assess the joint effects of sex steroid hormone concentrations and MD on breast cancer risk in premenopausal women.
Electronic supplementary material
(DOCX 20 kb)
Acknowledgements
This work was supported by the Breast Cancer Research Foundation and the National Cancer Institute (CA124865, CA67262, CA176726, and CA168504). K.A.B. was supported by the Nutritional Epidemiology of Cancer Education and Career Development Program (R25 CA098566) and the Simeon J. Fortin Charitable Foundation, Bank of America, Co-Trustee, N.A. We would like to thank the participants and staff of the Nurses’ Health Study II for their valuable contributions as well as the following state cancer registries for their help: AL, AZ, AR, CA, CO, CT, DE, FL, GA, ID, IL, IN, IA, KY, LA, ME, MD, MA, MI, NE, NH, NJ, NY, NC, ND, OH, OK, OR, PA, RI, SC, TN, TX, VA, WA, and WY. We also thank Kristina Astone for technical help. The authors assume full responsibility for analyses and interpretation of these data.
Abbreviations
- DHEA
Dehydroepiandrosterone
- DHEAS
Dehydroepiandrosterone sulfate
- SHBG
Sex hormone–binding globulin
- AMH
Anti-Müllerian hormone
- NHSII
Nurses’ Health Study II
- MD
Mammographic density
- BMI
Body mass index
- OR
Odds ratio
- CI
Confidence interval
References
- 1.Endogenous H, Breast Cancer Collaborative G. Key TJ, Appleby PN, Reeves GK, Travis RC, Alberg AJ, Barricarte A, Berrino F, Krogh V, et al. Sex hormones and risk of breast cancer in premenopausal women: a collaborative reanalysis of individual participant data from seven prospective studies. Lancet Oncol. 2013;14(10):1009–1019. doi: 10.1016/S1470-2045(13)70301-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kaaks R, Tikk K, Sookthai D, Schock H, Johnson T, Tjønneland A, Olsen A, Overvad K, Clavel-Chapelon F, Dossus L, Baglietto L, Rinaldi S, Chajes V, Romieu I, Boeing H, Schütze M, Trichopoulou A, Lagiou P, Trichopoulos D, Palli D, Sieri S, Tumino R, Ricceri F, Mattiello A, Buckland G, Ramón Quirós J, Sánchez MJ, Amiano P, Chirlaque MD, Barricarte A, Bas Bueno-de-Mesquita H, van Gils CH, Peeters PH, Andersson A, Sund M, Weiderpass E, Khaw KT, Wareham N, Key TJ, Travis RC, Merritt MA, Gunter MJ, Riboli E, Lukanova A. Premenopausal serum sex hormone levels in relation to breast cancer risk, overall and by hormone receptor status—results from the EPIC cohort. Int J Cancer. 2014;134(8):1947–1957. doi: 10.1002/ijc.28528. [DOI] [PubMed] [Google Scholar]
- 3.Fortner RT, Eliassen AH, Spiegelman D, Willett WC, Barbieri RL, Hankinson SE. Premenopausal endogenous steroid hormones and breast cancer risk: results from the Nurses’ Health Study II. Breast Cancer Res. 2013;15(2):R19. doi: 10.1186/bcr3394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McCormack VA, dos Santos Silva I. Breast density and parenchymal patterns as markers of breast cancer risk: a meta-analysis. Cancer Epidemiol Biomark Prev. 2006;15(6):1159–1169. doi: 10.1158/1055-9965.EPI-06-0034. [DOI] [PubMed] [Google Scholar]
- 5.Martin LJ, Boyd NF. Mammographic density. Potential mechanisms of breast cancer risk associated with mammographic density: hypotheses based on epidemiological evidence. Breast Cancer Res. 2008;10(1):201. doi: 10.1186/bcr1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boyd NF, Stone J, Martin LJ, Jong R, Fishell E, Yaffe M, Hammond G, Minkin S. The association of breast mitogens with mammographic densities. Br J Cancer. 2002;87(8):876–882. doi: 10.1038/sj.bjc.6600537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aiello EJ, Tworoger SS, Yasui Y, Stanczyk FZ, Potter J, Ulrich CM, Irwin M, McTiernan A. Associations among circulating sex hormones, insulin-like growth factor, lipids, and mammographic density in postmenopausal women. Cancer Epidemiol Biomark Prev. 2005;14(6):1411–1417. doi: 10.1158/1055-9965.EPI-04-0920. [DOI] [PubMed] [Google Scholar]
- 8.Tamimi RM, Hankinson SE, Colditz GA, Byrne C. Endogenous sex hormone levels and mammographic density among postmenopausal women. Cancer Epidemiol Biomark Prev. 2005;14(11 Pt 1):2641–2647. doi: 10.1158/1055-9965.EPI-05-0558. [DOI] [PubMed] [Google Scholar]
- 9.Greendale GA, Palla SL, Ursin G, Laughlin GA, Crandall C, Pike MC, Reboussin BA. The association of endogenous sex steroids and sex steroid binding proteins with mammographic density: results from the postmenopausal estrogen/progestin interventions mammographic density study. Am J Epidemiol. 2005;162(9):826–834. doi: 10.1093/aje/kwi286. [DOI] [PubMed] [Google Scholar]
- 10.Warren R, Skinner J, Sala E, Denton E, Dowsett M, Folkerd E, Healey CS, Dunning A, Doody D, Ponder B, et al. Associations among mammographic density, circulating sex hormones, and polymorphisms in sex hormone metabolism genes in postmenopausal women. Cancer Epidemiol Biomark Prev. 2006;15(8):1502–1508. doi: 10.1158/1055-9965.EPI-05-0828. [DOI] [PubMed] [Google Scholar]
- 11.Bertrand KA, Eliassen AH, Hankinson SE, Gierach GL, Xu X, Rosner B, Ziegler RG, Tamimi RM. Urinary estrogens and estrogen metabolites and mammographic density in premenopausal women. Breast Cancer Res Treat. 2012;136(1):277–287. doi: 10.1007/s10549-012-2240-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gierach GL, Patel DA, Falk RT, Pfeiffer RM, Geller BM, Vacek PM, Weaver DL, Chicoine RE, Shepherd JA, Mahmoudzadeh AP, Wang J, Fan B, Herschorn SD, Xu X, Veenstra T, Fuhrman B, Sherman ME, Brinton LA. Relationship of serum estrogens and metabolites with area and volume mammographic densities. Horm Cancer. 2015;6(2–3):107–119. doi: 10.1007/s12672-015-0216-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Walker K, Fletcher O, Johnson N, Coupland B, McCormack VA, Folkerd E, Gibson L, Hillier SG, Holly JM, Moss S, Dowsett M, Peto J, dos Santos Silva I. Premenopausal mammographic density in relation to cyclic variations in endogenous sex hormone levels, prolactin, and insulin-like growth factors. Cancer Res. 2009;69(16):6490–6499. doi: 10.1158/0008-5472.CAN-09-0280. [DOI] [PubMed] [Google Scholar]
- 14.Jung S, Stanczyk FZ, Egleston BL, Snetselaar LG, Stevens VJ, Shepherd JA, Van Horn L, LeBlanc ES, Paris K, Klifa C, Dorgan JF. Endogenous sex hormones and breast density in young women. Cancer Epidemiol Biomark Prev. 2015;24(2):369–378. doi: 10.1158/1055-9965.EPI-14-0939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yong M, Atkinson C, Newton KM, Aiello Bowles EJ, Stanczyk FZ, Westerlind KC, Holt VL, Schwartz SM, Leisenring WM, Lampe JW. Associations between endogenous sex hormone levels and mammographic and bone densities in premenopausal women. Cancer Causes Control. 2009;20(7):1039–1053. doi: 10.1007/s10552-009-9321-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Iversen A, Frydenberg H, Furberg AS, Flote VG, Finstad SE, McTiernan A, Ursin G, Wilsgaard T, Ellison PT, Jasienska G, Thune I. Cyclic endogenous estrogen and progesterone vary by mammographic density phenotypes in premenopausal women. Eur J Cancer Prev. 2016;25(1):9–18. doi: 10.1097/CEJ.0000000000000130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Noh JJ, Maskarinec G, Pagano I, Cheung LW, Stanczyk FZ. Mammographic densities and circulating hormones: a cross-sectional study in premenopausal women. Breast. 2006;15(1):20–28. doi: 10.1016/j.breast.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 18.Borugian MJ, Spinelli JJ, Gordon PB, Abanto Z, Brooks-Wilson A, Pollak MN, Warren LJ, Hislop TG, Gallagher RP. Fasting insulin and endogenous hormones in relation to premenopausal breast density (Canada) Cancer Causes Control. 2014;25(3):385–394. doi: 10.1007/s10552-014-0339-9. [DOI] [PubMed] [Google Scholar]
- 19.Jung S, Egleston BL, Chandler DW, Van Horn L, Hylton NM, Klifa CC, Lasser NL, LeBlanc ES, Paris K, Shepherd JA, Snetselaar LG, Stanczyk FZ, Stevens VJ, Dorgan JF. Adolescent endogenous sex hormones and breast density in early adulthood. Breast Cancer Res. 2015;17(1):77. doi: 10.1186/s13058-015-0581-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bertrand KA, Baer HJ, Orav EJ, Klifa C, Kumar A, Hylton NM, LeBlanc ES, Snetselaar LG, Van Horn L, Dorgan JF. Early life body fatness, serum anti-Mullerian hormone, and breast density in young adult women. Cancer Epidemiol Biomark Prev. 2016;25(7):1151–1157. doi: 10.1158/1055-9965.EPI-16-0185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dorgan JF, Stanczyk FZ, Egleston BL, Kahle LL, Shaw CM, Spittle CS, Godwin AK, Brinton LA. Prospective case–control study of serum Mullerian inhibiting substance and breast cancer risk. J Natl Cancer Inst. 2009;101(21):1501–1509. doi: 10.1093/jnci/djp331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nichols HB, Baird DD, Stanczyk FZ, Steiner AZ, Troester MA, Whitworth KW, Sandler DP. Anti-Müllerian hormone concentrations in premenopausal women and breast cancer risk. Cancer Prev Res (Phila) 2015;8(6):528–534. doi: 10.1158/1940-6207.CAPR-14-0377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eliassen AH, Zeleniuch-Jacquotte A, Rosner B, Hankinson SE. Plasma anti-Mullerian hormone concentrations and risk of breast cancer among premenopausal women in the Nurses’ health studies. Cancer Epidemiol Biomark Prev. 2016;25(5):854–860. doi: 10.1158/1055-9965.EPI-15-1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Eliassen AH, Missmer SA, Tworoger SS, Spiegelman D, Barbieri RL, Dowsett M, Hankinson SE. Endogenous steroid hormone concentrations and risk of breast cancer among premenopausal women. J Natl Cancer Inst. 2006;98(19):1406–1415. doi: 10.1093/jnci/djj376. [DOI] [PubMed] [Google Scholar]
- 25.Tworoger SS, Sluss P, Hankinson SE. Association between plasma prolactin concentrations and risk of breast cancer among predominately premenopausal women. Cancer Res. 2006;66(4):2476–2482. doi: 10.1158/0008-5472.CAN-05-3369. [DOI] [PubMed] [Google Scholar]
- 26.Tworoger SS, Missmer SA, Eliassen AH, Spiegelman D, Folkerd E, Dowsett M, Barbieri RL, Hankinson SE. The association of plasma DHEA and DHEA sulfate with breast cancer risk in predominantly premenopausal women. Cancer Epidemiol Biomark Prev. 2006;15(5):967–971. doi: 10.1158/1055-9965.EPI-05-0976. [DOI] [PubMed] [Google Scholar]
- 27.Byng JW, Boyd NF, Little L, Lockwood G, Fishell E, Jong RA, Yaffe MJ. Symmetry of projection in the quantitative analysis of mammographic images. Eur J Cancer Prev. 1996;5(5):319–327. doi: 10.1097/00008469-199610000-00003. [DOI] [PubMed] [Google Scholar]
- 28.Pettersson A, Hankinson SE, Willett WC, Lagiou P, Trichopoulos D, Tamimi RM. Nondense mammographic area and risk of breast cancer. Breast Cancer Res. 2011;13(5):R100. doi: 10.1186/bcr3041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rosner B, Cook N, Portman R, Daniels S, Falkner B. Determination of blood pressure percentiles in normal-weight children: some methodological issues. Am J Epidemiol. 2008;167(6):653–666. doi: 10.1093/aje/kwm348. [DOI] [PubMed] [Google Scholar]
- 30.Byrne C, Colditz GA, Willett WC, Speizer FE, Pollak M, Hankinson SE. Plasma insulin-like growth factor (IGF) I, IGF-binding protein 3, and mammographic density. Cancer Res. 2000;60(14):3744–3748. [PubMed] [Google Scholar]
- 31.Stone J, Ding J, Warren RM, Duffy SW, Hopper JL. Using mammographic density to predict breast cancer risk: dense area or percentage dense area. Breast Cancer Res. 2010;12(6):R97. doi: 10.1186/bcr2778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lokate M, Peeters PH, Peelen LM, Haars G, Veldhuis WB, van Gils CH. Mammographic density and breast cancer risk: the role of the fat surrounding the fibroglandular tissue. Breast Cancer Res. 2011;13(5):R103. doi: 10.1186/bcr3044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pettersson A, Graff RE, Ursin G, Santos Silva ID, McCormack V, Baglietto L, Vachon C, Bakker MF, Giles GG, Chia KS et al (2014) Mammographic density phenotypes and risk of breast cancer: a meta-analysis. J Natl Cancer Inst [DOI] [PMC free article] [PubMed]
- 34.Hankinson SE, Willett WC, Manson JE, Colditz GA, Hunter DJ, Spiegelman D, Barbieri RL, Speizer FE. Plasma sex steroid hormone levels and risk of breast cancer in postmenopausal women. J Natl Cancer Inst. 1998;90(17):1292–1299. doi: 10.1093/jnci/90.17.1292. [DOI] [PubMed] [Google Scholar]
- 35.Missmer SA, Eliassen AH, Barbieri RL, Hankinson SE. Endogenous estrogen, androgen, and progesterone concentrations and breast cancer risk among postmenopausal women. J Natl Cancer Inst. 2004;96(24):1856–1865. doi: 10.1093/jnci/djh336. [DOI] [PubMed] [Google Scholar]
- 36.Hirko KA, Spiegelman D, Willett WC, Hankinson SE, Eliassen AH. Alcohol consumption in relation to plasma sex hormones, prolactin, and sex hormone-binding globulin in premenopausal women. Cancer Epidemiol Biomark Prev. 2014;23(12):2943–2953. doi: 10.1158/1055-9965.EPI-14-0982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Södergård R, Bäckström T, Shanbhag V, Carstensen H. Calculation of free and bound fractions of testosterone and estradiol-17 beta to human plasma proteins at body temperature. J Steroid Biochem. 1982;16(6):801–810. doi: 10.1016/0022-4731(82)90038-3. [DOI] [PubMed] [Google Scholar]
- 38.Missmer SA, Spiegelman D, Bertone-Johnson ER, Barbieri RL, Pollak MN, Hankinson SE. Reproducibility of plasma steroid hormones, prolactin, and insulin-like growth factor levels among premenopausal women over a 2- to 3-year period. Cancer Epidemiol Biomark Prev. 2006;15(5):972–978. doi: 10.1158/1055-9965.EPI-05-0848. [DOI] [PubMed] [Google Scholar]
- 39.Vachon CM, Kuni CC, Anderson K, Anderson VE, Sellers TA. Association of mammographically defined percent breast density with epidemiologic risk factors for breast cancer (United States) Cancer Causes Control. 2000;11(7):653–662. doi: 10.1023/A:1008926607428. [DOI] [PubMed] [Google Scholar]
- 40.Boyd NF, Lockwood GA, Byng JW, Little LE, Yaffe MJ, Tritchler DL. The relationship of anthropometric measures to radiological features of the breast in premenopausal women. Br J Cancer. 1998;78(9):1233–1238. doi: 10.1038/bjc.1998.660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Samimi G, Colditz GA, Baer HJ, Tamimi RM. Measures of energy balance and mammographic density in the Nurses’ Health Study. Breast Cancer Res Treat. 2008;109(1):113–122. doi: 10.1007/s10549-007-9631-7. [DOI] [PubMed] [Google Scholar]
- 42.Durrleman S, Simon R. Flexible regression models with cubic splines. Stat Med. 1989;8(5):551–561. doi: 10.1002/sim.4780080504. [DOI] [PubMed] [Google Scholar]
- 43.Linton L, Taylor M, Dunn S, Martin L, Chavez S, Stanitz G, Huszti E, Minkin S, Boyd N. Associations of serum levels of sex hormones in follicular and luteal phases of the menstrual cycle with breast tissue characteristics in young women. PLoS One. 2016;11(10):e0163865. doi: 10.1371/journal.pone.0163865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Somboonporn W, Davis SR, National H, Medical Research C. Testosterone effects on the breast: implications for testosterone therapy for women. Endocr Rev. 2004;25(3):374–388. doi: 10.1210/er.2003-0016. [DOI] [PubMed] [Google Scholar]
- 45.Pasquali R, Vicennati V, Bertazzo D, Casimirri F, Pascal G, Tortelli O, Labate AM. Determinants of sex hormone-binding globulin blood concentrations in premenopausal and postmenopausal women with different estrogen status. Virgilio-menopause-health group. Metabolism. 1997;46(1):5–9. doi: 10.1016/S0026-0495(97)90159-1. [DOI] [PubMed] [Google Scholar]
- 46.Dupont J, Le Roith D. Insulin-like growth factor 1 and oestradiol promote cell proliferation of MCF-7 breast cancer cells: new insights into their synergistic effects. Mol Pathol. 2001;54(3):149–154. doi: 10.1136/mp.54.3.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hamelers IH, Steenbergh PH. Interactions between estrogen and insulin-like growth factor signaling pathways in human breast tumor cells. Endocr Relat Cancer. 2003;10(2):331–345. doi: 10.1677/erc.0.0100331. [DOI] [PubMed] [Google Scholar]
- 48.Frydenberg H, Flote VG, Iversen A, Finstad SE, Furberg AS, Torjesen PA, Wilsgaard T, Schlichting E, Ellison PT, Ursin G, Thune I. Insulin-like growth factor-1, growth hormone, and daily cycling estrogen are associated with mammographic density in premenopausal women. Cancer Causes Control. 2014;25(7):891–903. doi: 10.1007/s10552-014-0389-z. [DOI] [PubMed] [Google Scholar]
- 49.Schernhammer ES, Tworoger SS, Eliassen AH, Missmer SA, Holly JM, Pollak MN, Hankinson SE. Body shape throughout life and correlations with IGFs and GH. Endocr Relat Cancer. 2007;14(3):721–732. doi: 10.1677/ERC-06-0080. [DOI] [PubMed] [Google Scholar]
- 50.Rice MS, Tamimi RM, Connolly JL, Collins LC, Shen D, Pollak MN, Rosner B, Hankinson SE, Tworoger SS. Insulin-like growth factor-1, insulin-like growth factor binding protein-3 and lobule type in the Nurses’ Health Study II. Breast Cancer Res. 2012;14(2):R44. doi: 10.1186/bcr3141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Graham SJ, Stanchev PL, Lloyd-Smith JO, Bronskill MJ, Plewes DB. Changes in fibroglandular volume and water content of breast tissue during the menstrual cycle observed by MR imaging at 1.5 T. J Magn Reson Imaging. 1995;5(6):695–701. doi: 10.1002/jmri.1880050613. [DOI] [PubMed] [Google Scholar]
- 52.White E, Velentgas P, Mandelson MT, Lehman CD, Elmore JG, Porter P, Yasui Y, Taplin SH. Variation in mammographic breast density by time in menstrual cycle among women aged 40-49 years. J Natl Cancer Inst. 1998;90(12):906–910. doi: 10.1093/jnci/90.12.906. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 20 kb)