Abstract
Diisocyanates are highly reactive electrophiles utilized in the manufacture of a wide range of polyurethane products and have been identified as causative agents of occupational allergic respiratory disease. However, in spite of the significant occupational health burden associated with diisocyanate-induced asthma, the mechanism of disease pathogenesis remains largely unknown.
To better understand the fate of inhaled diisocyanates, a nose-only aerosol exposure system was constructed and utilized to expose a BALB/c mouse model to an aerosol generated from 4,4’-methylene diphenyl diisocyanate (MDI). Tissue and bronchoalveolar lavage samples were evaluated 4 and 24 h post-exposure for evidence of diisocyanate-protein haptenation, and a label-free quantitative proteomics strategy was employed to evaluate relative changes to the protein content of the cellular fraction of the lavage fluid.
Following MDI aerosol exposure, expression of the number of proteins with immunological or xenobiotic metabolism relevance is increased, including endoplasmin, cytochrome P450 and argininosuccinate synthase. Western blot analysis indicated MDI-conjugated protein in the lavage fluid, which was identified as serum albumin.
Tandem mass spectrometry analysis of MDI-albumin revealed MDI conjugation occurs at a dilysine motif at Lys525, as well as at a glutamine-lysine motif at Lys414, in good agreement with previously published in vitro data on diisocyanate-conjugated serum albumin.
Keywords: Aerosol, asthma, diisocyanate, mass spectrometry, proteomics
Introduction
Diisocyanates are low molecular weight chemicals utilized as cross-linkers in polyurethane production. 4,4′-methylene diphenyl diisocyanate (MDI) is the most widely used diisocyanate, accounting for more than 60% of the global diisocyanate market (Allport et al., 2003). MDI is widely used in the manufacture of such products as rigid polyurethane spray foam insulation, truck bed liners, wood products and adhesives. Because of its low vapor pressure (5 × 10−6 mm Hg), MDI presents less of an inhalation hazard than the more volatile diisocyanates toluene diisocyanate (TDI) or hexamethylene diisocyanate (HDI); however, MDI is a potent lung and dermal sensitizer and a major cause of occupational asthma worldwide (NIOSH, 2004). This low volatility has, in part, driven the industry towards the use of the “safer” MDI rather than the more volatile diisocyanate, TDI. However, in many applications, MDI is heated and/or aerosolized via a spray gun, thus creating respirable vapor and/or aerosols. Furthermore, many hot applications and thermal processes such as fires can release free MDI fumes from products. However, after heating, MDI tends to recondense in the air at ambient temperatures (Karoly et al., 2004). Because MDI hydrolyzes slowly in the atmosphere (the half-life of MDI in the air is 0.92 d), exposures to aerosolized MDI in the workplace are likely to be to small particulates containing free isocyanate. The major occupational hazards to workers from MDI exposure are assumed to be MDI liquid, vapor, aerosol and MDI-coated particles such as wood dust (Woellner et al., 1997).
Early recognition of occupational asthma and timely control of exposures has been associated with a lower risk of chronic irreversible respiratory effects, and biological monitoring of workers exposed to diisocyanates has been recommended (Wang & Petsonk, 2004). Although diisocyanate asthma is similar to other type I immune hypersensitivity disorders, in that asthmatics generally do not experience symptoms the first time they are exposed, and that repeated exposures over months and years tend to result in worsening symptoms, only a fraction of persons (44–55%) that develop diisocyanate asthma exhibit allergen-specific IgE. This complicates diagnosis in sick workers, as well as proactive screening and surveillance (Wisnewski & Jones, 2010). An alternative hypothesis is that repetitive cycles of injury and repair and oxidative stress contribute to diisocyanate asthma pathogenesis. Observation of “reactive airway disease syndrome” and asthma immediately following high dose exposures support the role of cytotoxicity in diisocyanate hypersensitivity with sensitization and asthma as secondary phenomena (Leroyer et al., 1998).
Although the burden of MDI-induced respiratory disease is well established, the molecular mechanism(s) of disease pathogenesis are poorly understood, in large part due to uncertainty regarding the antigenic form MDI takes in vivo (Wisnewski et al., 2010). The best current understanding of MDI toxicity involves MDI acting as a chemical hapten which reacts to covalently modify one or more “self” proteins at the site of exposure. This covalent modification causes antigenic changes in the structure/conformation of the native protein and subsequently elicits an immune response in the host. Which of these self-proteins are critical to pathogenesis have not been determined, although keratins (Hulst et al., 2015; Nayak et al., 2014) and serum albumin have been suggested. Several researchers have demonstrated by either immunological methods (Jin et al., 1993; Johannesson et al., 2001, 2004) or protein hydrolysis (Kumar et al., 2009; Sabbioni et al., 2010) that serum albumin can be modified in vivo by diisocyanates. However, none of these studies attempted to identify sequence-specific information on the site of modification. Previous studies in our laboratory have developed proteomics tools, in particular tandem mass spectrometry (Hettick et al., 2009) to investigate the reactive chemistry of the aromatic diisocyanates TDI (Chipinda et al., 2011; Hettick & Siegel, 2011, 2012; Hettick et al., 2012) and MDI (Hettick & Siegel, 2012; Mhike et al., 2013) in vitro with human serum albumin. Although these studies and those in other laboratories (Wisnewski et al., 2010) have identified a subset of likely biomarkers of diisocyanate exposure on serum albumin, thus far, attempts to identify site-specific albumin conjugation in vivo have failed (Luna et al., 2014).
A better understanding of the molecular basis for diisocyanate asthma is critical to improving biomonitoring, surveillance and patient outcomes. An international multidisciplinary team of researchers, clinicians and stakeholders met in 2013 to establish knowledge gaps and research priorities for isocyanates (Lockey et al., 2015). Among the identified research priorities were the following toxicology and biomarker-focused goals: [A] develop approaches to better understand the connections between isocyanate uptake, metabolism, immunogenicity and asthma pathogenesis, [B] further develop animal models to investigate the effects of different formulations, doses and routes of isocyanate exposure, [C] identify novel biomarkers of exposure, including biomarkers that identify the route of exposure (e.g. airway versus skin exposure), and also better biomarkers of effect (e.g. isocyanate sensitization and asthma). The current study is directly responsive to these stated research priorities and seeks to fill knowledge gaps in our current understanding of diisocyanate asthma by developing a murine model of MDI aerosol exposure. To better characterize the biochemical fate of inhaled diisocyanate particulates, a nose-only aerosol exposure system was constructed and utilized to expose a BALB/c mouse model to an aerosol generated from MDI. Tissue and bronchoalveolar lavage samples were evaluated 4 and 24 h post-exposure for identification and characterization of in vivo-formed MDI-protein haptenated complexes. Furthermore, a tandem mass spectrometry-based label-free quantitative proteomics strategy was employed to evaluate relative changes in protein expression of the cellular fraction of the lavage fluid to gain insight into changes in the cellular processes in the lung following MDI aerosol exposure.
Materials and methods
Chemicals and reagents
HPLC grade acetonitrile, HPLC grade formic acid, HPLC grade acetone, 3 Å molecular sieve (4–8 mesh), Tris-buffered saline, phosphate-buffered saline (PBS), Tween-20, bovine serum albumin, mouse serum albumin, porcine trypsin (proteomics grade), ammonium bicarbonate, tributylphosphine, iodoacetamide, trifluoroacetic acid (TFA) and 98% MDI were acquired from Sigma-Aldrich (St. Louis, MO). Radioimmunoprecipitation assay buffer (150 mm NaCl, 50 mm Tris-HCl [pH 7.5], 5.0 mm EDTA, 1.0 mm NaF, 1% sodium deoxycholate, 1% NP-40, 0.1% SDS and 0.25 mm phenylmethylsulfonyl fluoride), Imperial protein stain, T-PER tissue lysis buffer and 1-Step Ultra TMB blotting solution were acquired from Thermo Fisher Scientific (Waltham, MA). [Glu]1-Fibrinopeptide B was acquired from Protea Biosciences (Morgantown, WV). Laemmli sample buffer (62.5 mm Tris-HCl [pH 6.8], 2% SDS, 10% glycerol, 5% 2-mercaptoethanol, 0.004% bromphenol blue) was acquired from BioRad (Hercules, CA). Horseradish peroxidase conjugated antimouse IgG secondary antibody was acquired from Promega (Madison, WI). Fatal-Plus® (sodium pentobarbital) euthanasia solution was acquired from Vortech Pharmaceuticals, Ltd (Dearborn, MI). Mouse IgG1 isotype control clone MOPC-21 was acquired from BD Biosciences (San Jose, CA). Anti-MDI monoclonal antibody DA5 was prepared in house and has been previously described (Wisnewski & Liu, 2013). Dry acetone was prepared by incubating 10 ml HPLC grade acetone on 3 Å molecular sieve for a minimum of 24 h to adsorb water.
Animals
Female BALB/c mice were used in the murine models. BALB/c are an albino strain commonly used in investigations of cancer and immunology. This strain has been routinely used in our laboratory (Lemons et al., 2014; Nayak et al., 2014) and that of other researchers (Herrick et al., 2002, 2003; Wisnewski et al., 2015) to investigate diisocyanate allergy. Mice were purchased from Taconic (Germantown, NY) at 6–8 weeks-of-age. Upon arrival, the animals were allowed to acclimate for a minimum of 5 d and were randomly assigned to a treatment group. The animals were housed at a maximum of five/cage in ventilated plastic shoebox cages with hardwood chip bedding. NIH-31 modified 6% irradiated rodent diet (Teklad, Envigo, East Millstone, NJ) and tap water were provided from water bottles, ad libitum. Each animal cage was supplied with a 2.5 cm OD × 25 cm section of poly(vinyl chloride) pipe to facilitate acclimation of the animals to the restraint device. The animal facility temperature was maintained at 68–72 °F and the relative humidity between 36% and 57%. A light/dark cycle was maintained at 12-h intervals. Mice were euthanized at 4 or 24 h post-exposure. Mice were euthanized via intraperitoneal injection of sodium pentobarbital (200 mg/kg) followed by exsanguination upon a negative response to a toe pinch. Lungs were perfused with 10 ml icecold PBS, and bronchoalveolar lavage fluid (BALF) collected via 3 × 1 ml ice-cold PBS lavages. Trachea and lung were surgically removed and stored at −80 °C. Animals were housed in the Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC)-accredited NIOSH animal facility, and all procedures were performed in accordance with an animal protocol approved by the Institutional Animal Care and Use Committee.
Aerosol generation and exposure
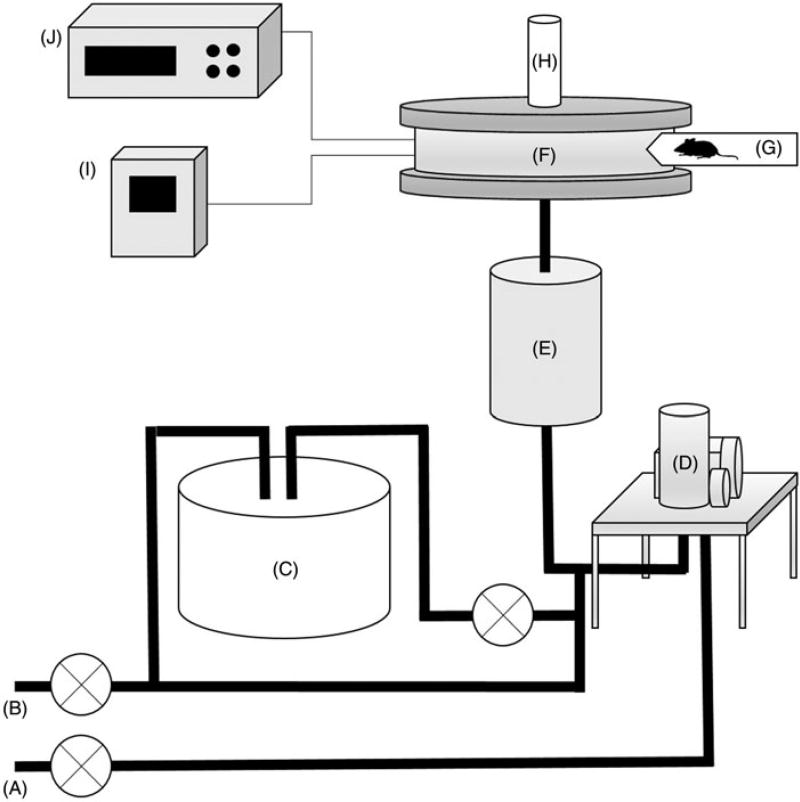
Prior to exposures, mice were acclimated to the nose-only restraint device with stays of increasing durations of 15, 30 and 60 min on consecutive days. Animal exposures were performed using an in-house constructed nose-only inhalation system, as depicted in Figure 1. Due to the tendency of MDI to hydrolyze to the dianiline or mixed polymerization products, even under proper storage conditions, we have determined that it is essential to ensure the relative purity of the MDI used for both conjugation and inhalation exposures. Although the monomeric MDI versus MDA can be assessed by gas chromatography/mass spectrometry (GC/MS), we have empirically determined that a simple qualitative assay is sufficient. Pure MDI will rapidly dissolve in dry acetone to yield a clear solution; however, degraded MDI will dissolve slowly or not at all, or will dissolve to yield a milky solution in dry acetone. The quality of the MDI was assessed prior to all experiments in this report. Immediately prior to exposures, MDI is twice-milled for 60 s in an electric laboratory grinder to produce a fine powder. The powder is packed into the large container of the Wright Dust Feeder (CH Technologies, Westwood, NJ) using a Benchtop Hydraulic Press to a pressure of 200 kg/cm2. Dry, filtered house air is supplied to the dust feeder, which is operated with the large scraping head at the maximum speed of 6 rpm. Diluent air and heated, humidified air are supplied as needed to maintain 50 ± 5% RH and 22 ± 2 °C. The aerosol-rich and diluent air are combined prior to entering a 20 l elutriation chamber, the exhaust of which is delivered to the inner plenum of the Nose-Only Inhalation Exposure System (NOIES, CH Technologies) where it is uniformly distributed to each animal through a trumpet at a flow rate of 0.7 L/min, as measured by a mass flow meter (GFM17, AALBORG, Orangeburg, NY) attached to one port of the manifold. The NOIES manifold can accommodate up to 12 animals per exposure. However, for the current study, five animals were exposed per experiment, and five ports of the manifold were plugged with rubber stoppers. The remaining two ports on the manifold were reserved for the mass flow meter and portable dust monitor (Series 1.100, GRIMM Aerosol Technik GmbH, Ainring, Germany), respectively. Concentration and particle size distribution of the MDI aerosol at the sampling aperture was monitored with the portable dust monitor. The exposure system was completely disassembled and thoroughly cleaned in between exposures. For safety, the entire aerosol exposure system is contained within a chemical fume hood. A single MDI aerosol exposure was performed for 60 min for each exposure group.
Figure 1.
Nose-only MDI aerosol inhalation exposure system. (A) House supply air. (B) Diluent air. (C) Heated humidification element. (D) Wright dust feeder. (E) 20 L elutriator. (F) Nose-only inhalation exposure manifold. (G) Mouse restraint. (H) HEPA exhaust filter. (I) Mass flow meter. (J) Portable dust monitor.
Preparation of MDI-albumin conjugate
MDI-mouse serum albumin conjugate (20:1 mol:mol ratio) was prepared as a positive control for both immunoprecipitation (IP) and Western blot experiments. A 10 ml conjugate stock solution was prepared by dissolving 0.5 mg mouse serum albumin in 9.7 ml distilled deionized water, to which was added 0.038 mg MDI in 300 µl dry acetone. The MDI/acetone solution was added slowly, in a dropwise fashion with constant mixing. The conjugate was used without further purification.
Tissue sample preparation
Lungs and tracheas were placed in 2.0 ml siliconized Eppendorf microfuge tubes (Fisher) with a 5 mm stainless steel bead (Qiagen, Valencia, CA) and 1.0 ml T-PER tissue lysis buffer. Tissues were disrupted by bead beating on a Tissuelyser (Qiagen) for three cycles of 2 min at 30 Hz. In between bead beating cycles, samples were cooled on ice for 3 min. Following bead beating, insoluble material was pelleted by centrifuging the samples for 20 min at 21 000× g at 4°C. The supernatant was removed and protein concentration determined at 280 nm using a Nanodrop 2000 (Thermo Scientific). The insoluble pellet was further fractionated by adding 100 µl Laemmli buffer and vigorously mixing at room temperature.
SDS-PAGE and Western blot
Protein samples (20 µg) were separated by SDS-PAGE on 4–20% 1.5 mm precast gels (BioRad) at 100V for 1.5 h. SDSPAGE bands were visualized with Imperial stain according to manufacturer’s instructions prior to downstream proteomic analysis. Western blot was performed by transferring SDS-PAGE separated protein bands to a 0.2 µm nitrocellulose membrane (BioRad) at 350 mA for 1 h at 4 °C. The membrane was washed three times for 5 min with 10 ml of tris-buffered saline containing 0.05% Tween-20 (TBST) on an orbital shaker at room temperature. Non-specific binding to the membrane was blocked by incubating with 10 ml 5% non-fat milk in TBST (W/V) for 1 h at RT. Following three five min washes with 10 ml TBST, the membrane was incubated with 0.5 µg/ml of DA5 anti-MDI monoclonal antibody in 5% BSA/TBST (W/V) at 4 °C overnight. Following primary antibody incubation, the membrane was washed three times with 10 ml TBST for 5 min and subsequently incubated with HRP-conjugated anti-mouse IgG secondary antibody at 1:5000 dilution in 5% non-fat milk/TBST (W/V) for 1 h at RT. Following secondary antibody incubation, the membrane was washed three times with 10 ml TBST for 5 min and the MDI-conjugated proteins were developed by incubation with 15 ml of Pierce™ 1-Step Ultra TMB Blotting Solution at RT.
In-gel trypsin digestion
Following SDS-PAGE, either specific bands of interest or entire gel lanes were excised and cut into 38 identical 1.5 × 5 mm bands using the OneTouch GridCutter (Gel Company, San Francisco, CA). Each band was deposited in a siliconized 0.6 ml microcentrifuge tube for in-gel trypsin digestion. All incubations were performed at 37 °C with shaking at 1000 rpm unless otherwise noted. Bands were destained twice for 30 min in 200 µl 200 mm NH4HCO3/40% acetonitrile, followed by dehydration for 30 min in a Savant DNA SpeedVac centrifugal concentrator (Thermo Scientific) at 43 °C. Bands were rehydrated with 100 µl 20 mm tributylphosphine in 25 mm NH4HCO3 and incubated for 15 min to reduce disulfide bonds. Cysteine residues were alkylated by incubating bands with 100 µl 40 mm iodoacetamide in 25 mm NH4HCO3 for 30 min. Bands were then washed twice with 200 µl 25 mm NH4HCO3 followed by one wash with 200 µl 25 mm NH4HCO3/50% acetonitrile. Bands were dehydrated 30 min in the centrifugal concentrator at 43 °C and rehydrated with 20 µl 20µg/ml trypsin solution in 40 mm NH4HCO3/9% acetonitrile at RT for 5 min. Following addition of 50 µl 40 mm NH4HCO3/9% acetonitrile, bands were allowed to digest overnight at 37 °C with shaking at 1000 rpm. After digestion, the supernatant was acidified by addition of 10 µl of 10% (vol) TFA to inactivate trypsin and taken immediately for ultra-performance liquid chromatography/qTOF-MSMS analysis.
Ultra-performance liquid chromatography
Enzymatic peptides were separated by UPLC using a nanoACQUITY system (Waters, Milford, MA) consisting of a sample manager, binary solvent manager and an auxiliary solvent pump. Samples were maintained at 4 °C in the sample manager prior to injection of a 4 µl aliquot which was trapped/desalted on a 5 µm SymmetryC18 (180 µm × 20 mm) trapping column with 95/5 A/B (A:0.1% formic acid; B:0.1% formic acid in acetonitrile) at a flow rate of 15 µl/min for 1 min. Analytical separation was performed on a 1.7 µm BEH130 C18 (75 µm × 150 mm) analytical column utilizing gradient elution at a flow rate of 300 nl/min and a gradient of 95/5 to 20/80 A/B over 50 min. The analytical column was maintained at 35 °C at all times.
Tandem mass spectrometry
The eluent from the UPLC system was directed to the nanoelectrospray source of a Waters SYNAPT MS quadrupole time-of-flight (qTOF) mass spectrometer. Positive ion nanoelectrospray was performed utilizing 10 µm PicoTip (Waters) emitters held at a potential of +2.5 kV. The cone voltage was held constant at +40V for all experiments. Dry N2 desolvation gas was supplied to the instrument at 110 psi via a nitrogen generator (NitroFlowLab, Parker Hannifin Corp., Haverhill, MA). [Glu]1-Fibrinopeptide B (100 fmol/µl in 90/10 A/B) was supplied to an orthogonal reference probe and the [M+2H]2+ ion (m/z=785.84265 u) measured as an external calibrant at 30 s intervals. Collision-induced dissociation was performed utilizing ultra-high purity argon as the collision gas. Spectra were acquired in a multiplexed or “MSe” fashion (Chakraborty et al., 2007). Briefly, alternating 1-s mass spectra are acquired. The first spectrum acquired at low (6 eV) collision energy allows high mass accuracy precursor ion mass measurement. The second spectrum acquired at high (25–40 eV ramp) collision energy allows high mass accuracy fragment ion mass measurement. The fragment ion spectra may be temporally correlated with precursor spectra post-run. This method of data acquisition allows all precursor ions to be fragmented and analyzed, rather than so-called “data dependent acquisition” methods that require real-time decisions to be made on which ions to select for fragmentation, and which may miss low-abundance precursor ions. MSe mass spectra were acquired from 5 to 60 min during the UPLC gradient.
Data analysis
Proteins were identified using ProteinLynx Global Server (PLGS) v2.5 (Waters) to search the mouse (Mus musculus) reference proteome available from UniProt (http://www.uniprot.org/proteomes/UP000000589). The database search was constrained as follows: mass error <25 ppm, protein molecular weights 0–250 kDa, digest reagent trypsin, missed cleavages 0–1 and carbamidomethyl cysteine as a fixed modification. The results of the PLGS search were exported to create a Microsoft Office Excel database for label-free quantitative analysis. Only proteins identified in each of three technical replicates were carried through to the final dataset. Label-free quantitation was performed by calculating the normalized spectral abundance factor (NSAF) for each protein, as given by Equation (1):
| (1) |
The NSAF for a protein k is the number of spectral counts (SpC, the total number of MS/MS spectra) identifying a protein k, divided by the protein’s length (L), divided by the sum of SpC/L for all N proteins in the dataset (Zybailov et al., 2006). Some mass spectra of interest were further analyzed using BioPharmaLynx v. 1.2 (Waters), a software program for comparative analysis of peptide mass maps and identification of sites of modification on known protein sequences. Default peptide mass map analysis criteria of 30 ppm mass error in both low and high collision energy mode were specified. Trypsin was specified as the digestion enzyme, and two missed cleavages were allowed. The submitted protein sequence was taken from P07724, “serum albumin, Mus musculus” (www.uniprot.org/uniprot/P07724) and the signal and propeptides (residues 1–24) removed. Custom modifiers were created for two bound forms of MDI. The first (MDI, C15H10N2O2, m/z=250.0742 u) represents MDI with both isocyanate moieties bound to a peptide via urea bonds. The second (MDI*, C14H12N2O, m/z=224.0950 u) represents one isocyanate moiety bound to a peptide via a urea bond, while the second isocyanate moiety is hydrolyzed to the primary amine. Identification of a potential MDI binding site proceeded via a rigorous procedure that involved the following steps: (1) Observation of a potential peptide-MDI conjugation product with less than 30 ppm m/Δm mass error in the exposed sample peptide mass map. (2) Comparison of exposed and control sample peptide mass maps from serum albumin shows that observed m/z and chromatographic retention time are unique to exposed sample. (3) MS/MS data contains bn-and yn-type ions consistent with the assigned sequence and modifier.
Results and discussion
Identification of MDI-conjugated protein following exposures
Groups of five female BALB/c mice were exposed to either house air or MDI aerosol using the NOIES apparatus. MDI concentrations of 4580 ± 1497 µg/m3 were achieved and maintained for each 60 min exposure. Of the total MDI particle load, roughly half, or 2243 ± 903.8 µg/m3, were of diameter less than 3.0 µm. Particles of less than 3.0 µm have an enhanced probability of deposition within the lower respiratory tract of the mouse (50–80%), with alveolar deposition approaching a maximum at diameters of approximately 1.0 µm (5–10%) (Schlesinger, 1985). The NOIES exposure apparatus was chosen for these experiments because it is specifically designed to expose only the breathing zone of the animals to the test article, MDI. Exposures were well tolerated by the animals, with no signs of visible distress or eye irritation observed. Upon removal from the NIOES apparatus, the animals immediately resumed normal feeding and grooming behaviors. The NIOSH recommended exposure limit (REL) for MDI is 0.05 mg/m3 (time-weighted average for a 10 h workday, 40-h workweek), so the current exposure represents the total MDI burden of approximately 100 h at the REL, or 10 work days. These exposures are approximately 15-fold below the immediately deadly to life and health threshold of 75 mg/m3 (NIOSH, 1997).
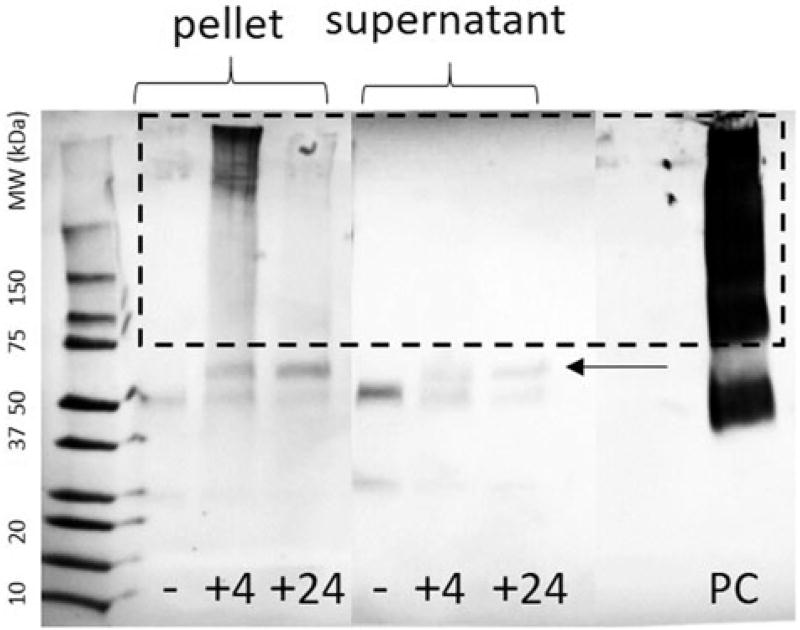
Immediately following euthanasia, lungs were perfused and lavaged, then lungs and trachea of the mice were surgically removed and mechanically disrupted by bead beating in tissue lysis buffer. Both the buffer-soluble and -insoluble fractions of the tissue lysate were analyzed by SDS-PAGE and Western blot using DA5 anti-MDI monoclonal antibody; however, both tissues tested negative for MDI-conjugated protein (see Supplemental Figures S1–S3). Subsequently, both the cell-free supernatant and the cellular fraction of the BALF were examined by SDS-PAGE anti-MDI Western blot, and the results are presented in Figure 2.
Figure 2.
SDS-PAGE anti-MDI Western blot of bronchoalveolar lavage fluid. BALF cell pellet lysate or supernatant from control air-exposed mice 4 h post-exposure and MDI-exposed mice 4- or 24-h post-exposure are indicated by “−, +4, +24”, respectively. Positive control MDI-conjugated mouse serum albumin (20:1 MDI:albumin mol ratio) is indicated by “PC”. Arrow indicates serum albumin band.
In both the cell-free supernatant and the cellular fraction of the BALF, the only bands which appear in the air-exposed control group are at approximately 25 and 50 kDa. These bands were excised, reduced, alkylated and subjected to in-gel trypsin digestion followed by UPLC-qTOF-MS/MS and were identified as IgG light and heavy chains, respectively, and are Western-blot positive as a result of the anti-mouse IgG secondary antibody utilized in the assay. In the MDI-exposed groups, however, a strong anti-MDI reactive band is observed at approximately 60 kDa which is absent in the air-exposed control group (indicated by an arrow in Figure 2). Following tandem mass spectrometry, these bands were identified as serum albumin. Interestingly, a high molecular weight “smear” of reactive protein is observed in the cellular fraction and positive control, but not the BALF supernatant (See dashed box in Figure 2). Similar streaking behavior has been well documented in in vitro experiments where serum albumin is exposed to relatively high (>10:1 diisocyanate:protein mole ratio) concentrations of diisocyanate, where a wide variety of mixed polymers are formed. Protein monomers may be crosslinked by a diisocyanate molecule, and diisocyanate may polymerize at one or more reactive amino acid sites on the protein (Hettick & Siegel, 2011, 2012; Hettick et al., 2012; Mhike et al., 2013). The positive control MDI-albumin conjugate in Figure 2 was prepared at a 20:1 MDI:protein ratio and demonstrates this behavior. In previous in vitro experiments, well-defined MDI-conjugated protein bands (such as observed in Figure 2) were only observed when diisocyanate:protein ratios are low (mol protein > mol diisocyanate) or when diisocyanate conjugation was mediated by transcarbamylation via glutathione (Wisnewski et al., 2011). Diisocyanates react reversibly with thiols, and glutathione is a tripeptide thiol antioxidant that is present in the lower respiratory tract at concentrations of more than 100 µM, and has been suggested to be the primary reactant of inhaled isocyanates (Lange et al., 1999) in a process which may be protective against MDI-protein conjugation (Wisnewski et al., 2005). It is not immediately evident why the cellular fraction of the BALF exhibits a high-molecular weight distribution of conjugated protein absent from the cell-free BALF supernatant, which is exposed to the highest load of MDI upon exposure. It is possible that inhaled MDI is reacting with glutathione (a reaction that is kinetically favored) followed by transcarbamylation of a single diisocyanate to albumin. Alternatively, cells may be more likely to take up highly-modified (damaged) proteins, leaving albumins that are only lightly modified and that more closely resemble self-protein. Finally, highly modified proteins could become insoluble, precipitate and be lost in the pellet. If MDI were crossing the cell barrier in an unreacted state, it seems reasonable that other intracellular proteins would be modified; however, upon excision and digestion of the high molecular weight “smear” observed in Figure 2 “+4”, serum albumin is the only protein identified upon subsequent tandem MS analysis.
Tandem mass spectrometry analysis was performed on the control and MDI-conjugated serum albumin bands excised from SDS-PAGE gels. Although more than 100 peptides were identified in both the control and exposed albumin bands (accounting for 100% and 99.8% sequence coverage, respectively), comparatively few demonstrated direct evidence of MDI conjugation. Previous studies in our laboratory have shown that even proteins extensively modified with diisocyanate yield comparatively poor tandem mass spectrometry data when compared to unmodified proteins (Hettick & Siegel, 2011, 2012; Hettick et al., 2012; Mhike et al., 2013). This is most likely due to the signal dilution that occurs when the limited ion current from a single tryptic peptide is divided among many potential reaction channels (e.g. inter- and intra-molecular crosslinking, hydrolysis of the isocyanate, polymerization of the isocyanate, etc.). In spite of this systematic limitation, we are able to determine two conjugation sites on serum albumin, Lys414 and Lys525. Both Lys414 and Lys525 are conserved in human albumin and have been previously identified as putative binding sites for both TDI and MDI based on in vitro tandem mass spectrometry studies (Hettick & Siegel, 2011, 2012).
Both of these sites are part of highly conserved albumin dilysine motifs, although in mouse, Lys414 occurs next to glutamine at position 413, rather than lysine (see Figure 3 for a sequence alignment between mouse and human serum albumin). These dilysine motifs have been previously hypothesized as possible target sites for diisocyanate conjugation in vitro (Hettick et al., 2012; Wisnewski et al., 2013) and human serum albumin conjugated at dilysine motifs has been recognized by serum of workers sensitized to MDI, suggesting this species may be immunologically active (Wisnewski et al., 2010).
Figure 3.
Sequence alignment of serum albumins. Mouse serum albumin (mus musculus, P07724) and human serum albumin (homo sapiens, P02768, in italics). MDI conjugation sites Lys414 and Lys524-525 are bolded.
Figure 4 presents a high-resolution peptide mass map mirror display comparing enzymatic peptides generated from either air-exposed control (top) or MDI-exposed (4-h post exposure, bottom) mouse serum albumin. This visualization tool reflects the control and exposed MS data about the m/z axis, allowing direct comparison of the peptide data. This data demonstrates that the unmodified tryptic peptide T61-62 (KQTALAELVK, m/z 1100.66 u) is observed for the control BALF, but not in the MDI-exposed BALF (Figure 4, Inset 1). However, m/z 1324.66 u representing the modified T61-62* peptide (K*QTALAELVK) is observable in the MDI-exposed BALF but not the control (Figure 4, Inset 2). This mass is consistent with the peptide [M+MDI*+H]+ ion, where MDI* (m/z 224.0950 u) is covalently bound to the amine nitrogen of the Lys525 sidechain. Furthermore, a second modified form of the T61-62* peptide is observed at m/z 1350.7421 u, consistent with an intramolecular crosslink formed by MDI (m/z 250.0742 u). Observation of the modified b2* fragment ion at m/z 507.24 u suggests that MDI forms an intramolecular crosslink between the amine groups on the sidechains of lysine and glutamine. A modified form of tryptic peptide T47-48 is observed (YTQKAPQVSTPT, m/z 1551.7357 u) which exhibits a modified b5* fragment ion, but not a b2* fragment ion, suggesting the MDI is located on the sequence “QKA”, and most reasonably exists as a crosslink between Glu413 and Lys414. This observation is in good agreement with a previous in vitro study, which suggested that the dilysine located at Lys413–414 in human albumin is a preferred diisocyanate binding target (Hettick & Siegel, 2012). In mouse, this site is not a dilysine as the residue at position 413 is a glutamine, not a lysine. Conjugation at this site in mouse suggests that the dilysine motif is not essential for the reaction. Although we previously hypothesized that sidechain interaction between the two lysines of a dilysine motif results in a lower pKa for the sidechain primary amine making reaction at that site more favorable, this data suggests that lysine and glutamine may be able to interact in a similar fashion to dilysine.
Figure 4.
High-resolution peptide mass map mirror display of mouse serum albumin. Control (top) and MDI-exposed (bottom) peptide mass maps are reflected about the m/z axis. Arrows indicate peptides at m/z (1) 1100.66 u and (2) 1324.66 u, the unmodified and MDI-conjugated forms of peptide T61-62, respectively.
Label-free quantitative proteomics of bronchoalveolar lavage fluid following exposures
In addition to characterizing the chemical haptenation of self-protein such as serum albumin following MDI exposure, the second goal of the present proteomics-based study was to examine changes in relative expression of the protein content of the cellular population of the BAL fluid in response to MDI exposure. In order to maximize sequence coverage across the broad range of protein molecular weights and concentrations, one-dimensional SDS-PAGE was chosen as a protein pre-fractionation method prior to tandem MS analysis. Such “GeLC-MS” methods are robust and reproducible for both protein identification and quantification (Dzieciatkowska et al., 2014). Tandem mass spectrometry-based protein identification resulted in the identification 398 (control group), 261 (4 h post-exposure group) and 253 (24 h post-exposure group) unique proteins, allowing for a direct comparison of 196 (control versus 4 h) and 203 (control versus 24 h) protein expression levels. Proteins were quantified using a label-free “NSAF” approach (Zybailov et al., 2006). An NSAF-based label-free quantitation approach has previously been demonstrated to produce the most reproducible results across both technical and biological replicates (McIlwain et al., 2012). Briefly, the NSAF is the number of peptides identified by tandem mass spectrometry, normalized to the length of the protein, and expressed as a fraction of the total normalized peptides identified across all proteins. NSAF values for individual proteins may then be compared across treatment groups. Proteins that exhibit a greater than two-fold change in NSAF are considered significant and are reported in Table 1 (control versus 4 h) and Table 2 (control versus 24 h). Although protein NSAF values corresponding to less than two-fold up- or down regulation have been demonstrated to be statistically significant, two-fold changes are commonly accepted reporting criteria in label-free quantitation studies (Zybailov et al., 2006).
Table 1.
Differentially expressed proteins identified in the cellular fraction of bronchoalveolar lavage fluid of mice exposed to MDI aerosol, 4 h post exposure.
| Accession | Description | MW (Da) | Fold change | Up/down |
|---|---|---|---|---|
| P33267 | Cytochrome P450 2F2 | 55 913 | +4.08 | Up |
| Q3UJ34 | Argininosuccinate synthase | 46 555 | +4.36 | Up |
| P08113 | Endoplasmin | 92 418 | +3.22 | Up |
| Q3U7H1 | Napsin A | 45 515 | +2.95 | Up |
| Q8CGP7 | Histone H2A | 14 140 | +2.89 | Up |
| Q64524 | Histone H2B | 13 984 | +2.62 | Up |
| Q542G9 | Annexin | 38 651 | +2.55 | Up |
| Q5FWJ3 | Vimentin | 53 655 | +2.44 | Up |
| P09041 | Phosphoglycerate kinase 2 | 44 824 | +2.43 | Up |
| D3YYR8 | Serotransferrin | 25 658 | +2.22 | Up |
| A0A087WRB4 | Tubulin alpha 4A | 11 094 | +2.12 | Up |
| Q9CPN9 | Protein 2210010C04Rik | 26 404 | +2.04 | Up |
| O08529 | Calpain 2 catalytic subunit | 79 821 | −2.02 | Down |
| P16858 | Glyceraldehyde 3 phosphate dehydrogenase | 35 787 | −2.07 | Down |
| A6PWS5 | Gelsolin | 28 042 | −2.09 | Down |
| Q8BSL7 | ADP ribosylation factor 2 | 20 732 | −2.33 | Down |
| P63101 | 14 3 3 protein zeta delta | 27 753 | −2.40 | Down |
| Q5SQ27 | MCG140354 | 46 054 | −2.90 | Down |
| P61205 | ADP ribosylation factor 3 | 20 587 | −2.97 | Down |
| V9GXA7 | Protein Gm15294 | 12 957 | −3.29 | Down |
| O08553 | Dihydropyrimidinase-related protein 2 | 62 238 | −3.33 | Down |
| P10107 | Annexin A1 | 38 709 | −3.47 | Down |
| D3YW48 | Calpain small subunit 1 | 25 294 | −5.80 | Down |
| O08709 | Periredoxin 6 | 24 854 | −6.09 | Down |
Table 2.
Differentially expressed proteins identified in the cellular fraction of bronchoalveolar lavage fluid of mice exposed to MDI aerosol, 24 h post exposure.
| Accession | Description | MW (Da) | Fold change | Up/Down |
|---|---|---|---|---|
| P33267 | Cytochrome P450 2F2 | 55 913 | +4.97 | Up |
| Q3UJ34 | Argininosuccinate synthase | 46 555 | +4.36 | Up |
| P08113 | Endoplasmin | 92 418 | +3.07 | Up |
| Q3U7H1 | Napsin A | 45 515 | +2.91 | Up |
| Q64524 | Histone H2B | 13 984 | +2.51 | Up |
| Q8CGP7 | Histone H2A | 14 140 | +2.44 | Up |
| P09041 | Phosphoglycerate kinase 2 | 44 824 | +2.43 | Up |
| Q9D7P7 | Chloride intracellular channel protein 3 | 26 828 | −2.01 | Down |
| P61205 | ADP ribosylation factor 3 | 20 587 | −2.03 | Down |
| Q63836 | Selenium binding protein 2 | 54 576 | −2.04 | Down |
| A0A0J9YUJ8 | Gelsolin | 18 727 | −2.10 | Down |
| Q9Z1R9 | MCG124046 | 26 117 | −2.23 | Down |
| O35945 | Aldehyde dehydrogenase | 54 552 | −2.30 | Down |
| D3YYI5 | Glyceraldehye 3 phosphate dehydrogenase | 36 280 | −2.30 | Down |
| O08529 | Calpain 2 | 79 821 | −2.35 | Down |
| P29699 | Alpha 2 HS glycoprotein | 37 301 | −2.36 | Down |
| A2AE89 | Glutathione S transferase | 23 520 | −2.55 | Down |
| O08553 | Dihydropyrimidinase-related protein 2 | 62 238 | −3.23 | Down |
| P10107 | Annexin A1 | 38 709 | −3.56 | Down |
| O88456 | Calpain small subunit | 28 444 | −12.0 | Down |
The most dramatically upregulated protein following aerosol exposure to MDI is cytochrome P450 2F2. Cytochrome P450 is a large protein superfamily of hemecontaining enzymes that metabolize a broad range of endogenous and exogenous compounds. The isoform identified here, cytochrome P450 2F2, is expressed in the respiratory tract and is active toward many pulmonary xenobiotic compounds including small organics such as naphthalene, styrene, benzene and more; however, the precise role that cytochrome P450 2F2 plays in chemical toxicity has been difficult to elucidate (Li et al., 2011). Cytochrome P450 2F2 is able to metabolically activate several of these toxicants to reactive epoxides, which results in lung injury (Buckpitt et al., 1995). To date, there are no known endogenous compounds metabolized by cytochrome P450 2F2 (Zhang & Ding, 2008). As a strong electrophile, the hazard of MDI exposure has long been considered its ability to haptenate self-protein; however, if MDI exposure is able to induce cytochrome P450 2F2 expression and is able to be metabolized to some reactive intermediate, that would warrant future mechanistic study.
Other proteins upregulated both 4 and 24 h post exposure include argininosuccinate synthase, a key component of the citrulline-NO cycle, that has been shown to be upregulated by pro-inflammatory cytokines such as IL-1β, TNF-α and IFN-γ (Husson et al., 2003). Of interest from an immunology perspective is the upregulation of endoplasmin, a versatile heat shock protein of the innate and adaptive immune systems. It can both bind and carry peptides to major histocompatibility complex class I proteins of the dendritic and other antigen presenting cells, but it can also function like a cytokine by inducing dendritic cells to produce IL-12 and TNF-α. As Schild described it, “they not only report the murder, they also provide a description of the suspect, thereby helping the immune system to discriminate between self and nonself, or between self and altered-self, respectively” (Schild & Rammensee, 2000). Only one protein that is significantly upregulated at 4 h post exposure is not still upregulated 24 h later, vimentin. Vimentin is an intermediate filament protein expressed in mesenchymal cells. It also plays a role in aggresome formation, forming a cage surrounding a core of aggregated protein. This has been proposed as a general response of cells that occurs when the capacity of the proteasome is exceeded by aggregation-prone misfolded protein (Johnston et al., 1998). In this experiment, relatively large micrometer-sized particulates of MDI are deposited in the airway. Based on the well-documented tendency of diisocyanates to form high molecular weight covalently crosslinked complexes with self-proteins, it is possible that the formation of such protein-MDI complexes or perhaps the MDI particles themselves, in the lung are recognized as non-self or damaged-self leading to vimentin aggresome formation as part of the acute host response.
Proteins downregulated in response to MDI aerosol exposure include those involved in actin assembly and cell mobility processes, such as chloride intracellular channel protein 3, ADP ribosylation factor 3, gelsolin, calpain, dihydropyrimidinase-related protein 2 and annexin. Furthermore, annexin A1 plays a role in anti-inflammatory processes. Other proteins, such as aldehyde dehydrogenase, selenium binding protein 2 and glutathione S transferase play a role in metabolism or detoxification of xenobiotics. Taken in total, the quantitative proteomics data suggest that in the 24 h immediately following an MDI aerosol respiratory exposure, cellular response processes such as inflammation, oxidative stress, detoxification and early immune processing are increased, whereas cell adhesion, mobility and actin assembly are decreased.
Conclusion
A nose-only dry aerosol exposure apparatus was designed and constructed to expose a BALB/c mouse model to respirable particulates of MDI. Following exposures, MDI-conjugated serum albumin hapten-protein complexes were identified in both the cellular fraction and cell-free supernatant of BALF. MDI conjugation was confirmed on Lys414 and Lys525, the second lysine of a dilysine motif conserved in human serum albumin. These represent the first site-specific determination of MDI conjugation to serum albumin from an in vivo study, and suggest possible candidate biomarkers that should be validated. Label-free quantitative proteomics of the cellular fraction of the BALF demonstrated a subset of up- and downregulated proteins, suggestive of increased inflammation, oxidative stress, chemical detoxification and early immune response processes. Taken in total, the current study is directly responsive to previously-established knowledge gaps and research priorities within the field of isocyanates and human health.
Supplementary Material
Acknowledgments
The findings and the conclusions in this report are those of the authors and do not necessarily represent the views of the National Institute for Occupational Safety and Health.
Footnotes
Disclosure of interest
No potential conflict of interest was reported by the authors.
References
- Allport DC, Gilbert DS, Outterside SM. MDI, TDI and the polyurethane industry. In: Allport DC, Gilbert DS, Outterside SM, editors. MDI & TDI: safety, health, and the environment. Chichester, England: John Wiley & Sons Ltd.; 2003. pp. 11–23. [Google Scholar]
- Buckpitt A, Chang AM, Weir A, et al. Relationship of cytochrome P450 activity to Clara cell cytotoxicity. IV. Metabolism of naphthalene and naphthalene oxide in microdissected airways from mice, rats, and hamsters. Mol Pharmacol. 1995;47:74–81. [PubMed] [Google Scholar]
- Chakraborty AB, Berger SJ, Gebler JC. Use of an integrated MS – multiplexed MS/MS data acquisition strategy for high-coverage peptide mapping studies. Rapid Commun Mass Spectrom. 2007;21:730–44. doi: 10.1002/rcm.2888. [DOI] [PubMed] [Google Scholar]
- Chipinda I, Hettick JM, Siegel PD. Haptenation: chemical reactivity and protein binding. J Allergy (Cairo) 2011;2011:839682. doi: 10.1155/2011/839682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dzieciatkowska M, Hill R, Hansen KC. GeLC-MS/MS analysis of complex protein mixtures. Methods Mol Biol. 2014;1156:53–66. doi: 10.1007/978-1-4939-0685-7_4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrick CA, Das J, Xu L, et al. Differential roles for CD4 and CD8 T cells after diisocyanate sensitization: genetic control of TH2- induced lung inflammation. J Allergy Clin Immunol. 2003;111:1087–94. doi: 10.1067/mai.2003.1413. [DOI] [PubMed] [Google Scholar]
- Herrick CA, Xu L, Wisnewski AV, et al. A novel mouse model of diisocyanate-induced asthma showing allergic-type inflammation in the lung after inhaled antigen challenge. J Allergy Clin Immunol. 2002;109:873–8. doi: 10.1067/mai.2002.123533. [DOI] [PubMed] [Google Scholar]
- Hettick JM, Ruwona TB, Siegel PD. Structural elucidation of isocyanate-peptide adducts using tandem mass spectrometry. J Am Soc Mass Spectrom. 2009;20:1567–75. doi: 10.1016/j.jasms.2009.04.016. [DOI] [PubMed] [Google Scholar]
- Hettick JM, Siegel PD. Determination of the toluene diisocyanate binding sites on human serum albumin by tandem mass spectrometry. Anal Biochem. 2011;414:232–8. doi: 10.1016/j.ab.2011.03.035. [DOI] [PubMed] [Google Scholar]
- Hettick JM, Siegel PD. Comparative analysis of aromatic diisocyanate conjugation to human albumin utilizing multiplexed tandem mass spectrometry. Int J Mass Spectrom. 2012;309:168–75. [Google Scholar]
- Hettick JM, Siegel PD, Green BJ, et al. Vapor conjugation of toluene diisocyanate to specific lysines of human albumin. Anal Biochem. 2012;421:706–11. doi: 10.1016/j.ab.2011.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulst AG, Verstappen DR, van der Riet-Van Oeveren D, et al. Mass spectrometric identification of isocyanate-induced modifications of keratins in human skin. Chem Biol Interact. 2015;237:141–50. doi: 10.1016/j.cbi.2015.06.008. [DOI] [PubMed] [Google Scholar]
- Husson A, Brasse-Lagnel C, Fairand A, et al. Argininosuccinate synthetase from the urea cycle to the citrulline-NO cycle. Eur J Biochem. 2003;270:1887–99. doi: 10.1046/j.1432-1033.2003.03559.x. [DOI] [PubMed] [Google Scholar]
- Jin R, Day BW, Karol MH. Toluene diisocyanate protein adducts in the bronchoalveolar lavage of guinea pigs exposed to vapors of the chemical. Chem Res Toxicol. 1993;6:906–12. doi: 10.1021/tx00036a023. [DOI] [PubMed] [Google Scholar]
- Johannesson G, Rosqvist S, Lindh CH, et al. Serum albumins are the major site for in vivo formation of hapten-carrier protein adducts in plasma from humans and guinea-pigs exposed to type-1 allergy inducing hexahydrophthalic anhydride. Clin Exp Allergy. 2001;31:1021–30. doi: 10.1046/j.1365-2222.2001.01109.x. [DOI] [PubMed] [Google Scholar]
- Johannesson G, Sennbro CJ, Willix P, et al. Identification and characterisation of adducts between serum albumin and 4,4′-methylenediphenyl diisocyanate (MDI) in human plasma. Arch Toxicol. 2004;78:378–83. doi: 10.1007/s00204-004-0555-2. [DOI] [PubMed] [Google Scholar]
- Johnston JA, Ward CL, Kopito RR. Aggresomes: a cellular response to misfolded proteins. J Cell Biol. 1998;143:1883–98. doi: 10.1083/jcb.143.7.1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karoly WJ, Flatley JJ, Stevenson RD, Bowers JD. Airborne concentrations of methylene diphenyl diisocyanate (MDI) in North American wood mills during the manufacturing of oriented strand board (OSB) J Occup Environ Hyg. 2004;1:789–98. doi: 10.1080/15459620490885644. [DOI] [PubMed] [Google Scholar]
- Kumar A, Dongari N, Sabbioni G. New isocyanate-specific albumin adducts of 4,4′-methylenediphenyl diisocyanate (MDI) in rats. Chem Res Toxicol. 2009;22:1975–83. doi: 10.1021/tx900270z. [DOI] [PubMed] [Google Scholar]
- Lange RW, Day BW, Lemus R, et al. Intracellular S-glutathionyl adducts in murine lung and human bronchoepithelial cells after exposure to diisocyanatotoluene. Chem Res Toxicol. 1999;12:931–6. doi: 10.1021/tx990045h. [DOI] [PubMed] [Google Scholar]
- Lemons AR, Siegel PD, Mhike M, et al. A murine monoclonal antibody with broad specificity for occupationally relevant diisocyanates. J Occup Environ Hyg. 2014;11:101–10. doi: 10.1080/15459624.2013.843783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leroyer C, Perfetti L, Cartier A, Malo JL. Can reactive airways dysfunction syndrome (RADS) transform into occupational asthma due to “sensitisation” to isocyanates? Thorax. 1998;53:152–3. doi: 10.1136/thx.53.2.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Wei Y, Van Winkle L, et al. Generation and characterization of a Cyp2f2-null mouse and studies on the role of CYP2F2 in naphthalene-induced toxicity in the lung and nasal olfactory mucosa. J Pharmacol Exp Ther. 2011;339:62–71. doi: 10.1124/jpet.111.184671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lockey JE, Redlich CA, Streicher R, et al. Isocyanates and human health: multistakeholder information needs and research priorities. J Occup Environ Med. 2015;57:44–51. doi: 10.1097/JOM.0000000000000278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luna LG, Green BJ, Zhang F, et al. Quantitation of 4,4′-methylene diphenyl diisocyanate human serum albumin adducts. Toxicol Rep. 2014;1:743–51. doi: 10.1016/j.toxrep.2014.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIlwain S, Mathews M, Bereman MS, et al. Estimating relative abundances of proteins from shotgun proteomics data. BMC Bioinformatics. 2012;13:308. doi: 10.1186/1471-2105-13-308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mhike M, Chipinda I, Hettick JM, et al. Characterization of methylene diphenyl diisocyanate-haptenated human serum albumin and hemoglobin. Anal Biochem. 2013;440:197–204. doi: 10.1016/j.ab.2013.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nayak AP, Hettick JM, Siegel PD, et al. Toluene diisocyanate (TDI) disposition and co-localization of immune cells in hair follicles. Toxicol Sci. 2014;140:327–37. doi: 10.1093/toxsci/kfu079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NIOSH. NIOSH pocket guide to chemical hazards. Cincinnati, OH: U.S. Department of Health and Human Services, Public Health Service, Centers for Disease Control and Prevention, National Institute for Occupational Safety and Health; 1997. [Google Scholar]
- NIOSH. A summary of health hazard evaluations: issues related to occupational exposure to isocyanates, 1989–2002. Cincinnati (OH): U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Institute for Occupational Safety and Health; 2004. [Google Scholar]
- Sabbioni G, Dongari N, Kumar A. Determination of a new biomarker in subjects exposed to 4,4′-methylenediphenyl diisocyanate. Biomarkers. 2010;15:508–15. doi: 10.3109/1354750X.2010.490880. [DOI] [PubMed] [Google Scholar]
- Schild H, Rammensee HG. gp96 – the immune system’s Swiss army knife. Nat Immunol. 2000;1:100–1. doi: 10.1038/77770. [DOI] [PubMed] [Google Scholar]
- Schlesinger RB. Comparative deposition of inhaled aerosols in experimental animals and humans: a review. J Toxicol Environ Health. 1985;15:197–214. doi: 10.1080/15287398509530647. [DOI] [PubMed] [Google Scholar]
- Wang ML, Petsonk EL. Symptom onset in the first 2 years of employment at a wood products plant using diisocyanates: some observations relevant to occupational medical screening. Am J Ind Med. 2004;46:226–33. doi: 10.1002/ajim.20050. [DOI] [PubMed] [Google Scholar]
- Wisnewski AV, Hettick JM, Siegel PD. Toluene diisocyanate reactivity with glutathione across a vapor/liquid interface and subsequent transcarbamoylation of human albumin. Chem Res Toxicol. 2011;24:1686–93. doi: 10.1021/tx2002433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisnewski AV, Jones M. Pro/Con debate: is occupational asthma induced by isocyanates an immunoglobulin E-mediated disease? Clin Exp Allergy. 2010;40:1155–62. doi: 10.1111/j.1365-2222.2010.03550.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisnewski AV, Liu J. Molecular determinants of humoral immune specificity for the occupational allergen, methylene diphenyl diisocyanate. Mol Immunol. 2013;54:233–7. doi: 10.1016/j.molimm.2012.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisnewski AV, Liu J, Colangelo CM. Glutathione reaction products with a chemical allergen, methylene-diphenyl diisocyanate, stimulate alternative macrophage activation and eosinophilic airway inflammation. Chem Res Toxicol. 2015;28:729–37. doi: 10.1021/tx5005002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisnewski AV, Liu J, Redlich CA. Antigenic changes in human albumin caused by reactivity with the occupational allergen diphenylmethane diisocyanate. Anal Biochem. 2010;400:251–8. doi: 10.1016/j.ab.2010.01.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisnewski AV, Liu Q, Liu J, Redlich CA. Glutathione protects human airway proteins and epithelial cells from isocyanates. Clin Exp Allergy. 2005;35:352–7. doi: 10.1111/j.1365-2222.2005.02185.x. [DOI] [PubMed] [Google Scholar]
- Wisnewski AV, Mhike M, Hettick JM, et al. Hexamethylene diisocyanate (HDI) vapor reactivity with glutathione and subsequent transfer to human albumin. Toxicol In Vitro. 2013;27:662–71. doi: 10.1016/j.tiv.2012.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woellner RC, Hall S, Greaves I, Schoenwetter WF. Epidemic of asthma in a wood products plant using methylene diphenyl diisocyanate. Am J Ind Med. 1997;31:56–63. doi: 10.1002/(sici)1097-0274(199701)31:1<56::aid-ajim9>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- Zhang Q-Y, Ding X. The CYP2F, CYP2G and CYP2J Subfamilies. In: Ioannides C, editor. Cytochromes P450: role in metabolism and toxicity of drugs and other xenobiotics. Cambridge (UK): RSC Publishing; 2008. pp. 309–53. [Google Scholar]
- Zybailov B, Mosley AL, Sardiu ME, et al. Statistical analysis of membrane proteome expression changes in Saccharomyces cerevisiae. J Proteome Res. 2006;5:2339–47. doi: 10.1021/pr060161n. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.