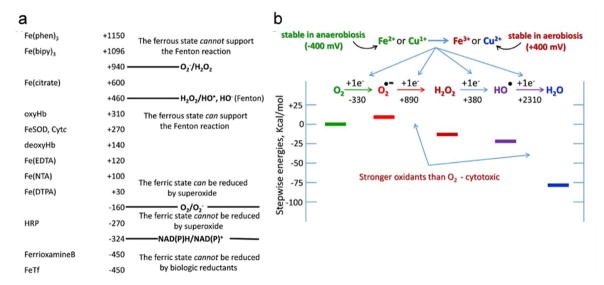
Fig. 1.
Iron and oxygen redox scales. (a) One-electron reduction potentials (in mV) of various iron complexes bracket the one-electron reduction potentials of oxygen and its 1e− reduction intermediates. The thermodynamically probable electron transfer events are indicated. Although not noted, typical copper complexes have potentials less than 100 mV and thus support these 1e− oxygen reduction reactions. (b) A thermodynamic scale of oxygen and its one-election reduction products. This diagram emphasizes the increasingly robust oxidation potential of oxygen’s 1e− reduced species.