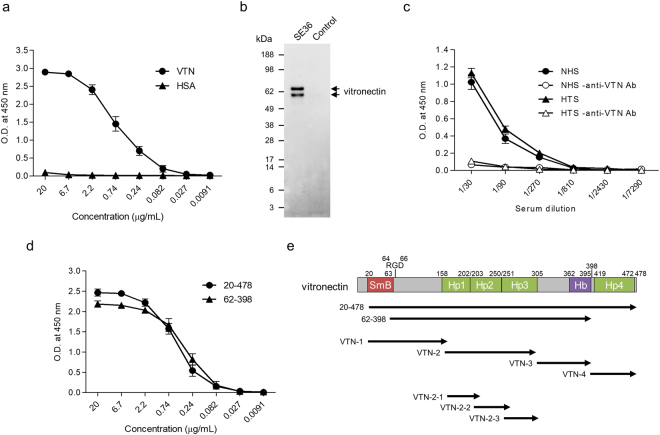
Figure 1.
Binding assay of vitronectin (VTN) to SE36. (a) Reactivity of the purified VTN and HSA against SE36. SE36 was adsorbed to microtiter plate and VTN or human serum albumin (HSA) was added at the indicated concentrations. (b) Western blotting of elutes from SE36-immobilized and control columns. The anti-VTN pAb detected two forms (V75 and V65; upper band and lower band, respectively) of VTN. Each elute was run in an SDS-PAGE gel and probed with anti-VTN pAb (diluted 1:2000). (c) Reactivity of VTN in naïve human serum (NHS) and Ugandan high anti-SE36 IgG-titer serum (HTS) against SE36. SE36 was adsorbed to microtiter plate and NHS or HTS was added at the indicated dilutions (closed symbols). Open symbols show reactions without anti-VTN Ab as negative control. (d) Reactivity of commercially available VTN recombinants (see also Fig. 1e) against SE36. SE36 was adsorbed to microtiter plate and VTN recombinants were added at the indicated concentrations. (e) Schematic representation of VTN. “SmB”, “Hp”, and “Hb” indicate Somatomedin-B motif, Hemopexin domain, and Heparin-binding region, respectively. The number 398 denotes the endogenous cleavage site. The designations “20–478” and “62–398” indicate commercially available VTN recombinants. VTN-1 to -4, as well as VTN-2-1 to -2-3 denote truncated recombinant forms. Results in (a, c, and d) are expressed as means ± SD from three independent experiments. In (c), statistical analysis between NHS and HTS was performed using a Mann-Whitney U test. No significant differences were found.