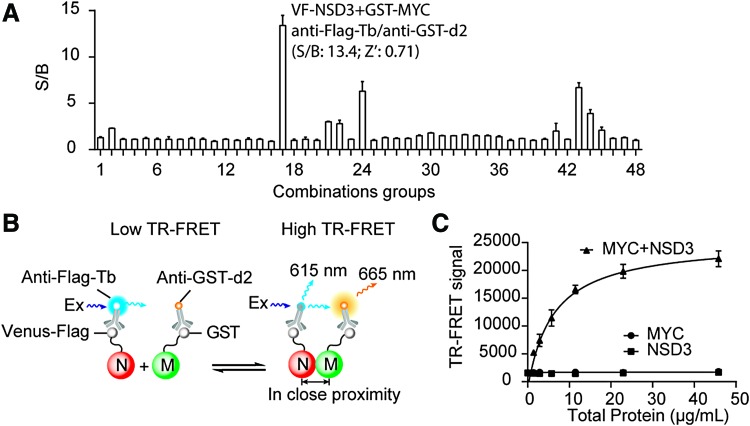
Fig. 1.
TR-FRET assay development for NSD3/MYC interaction. (A) Selection of constructs and TR-FRET antibodies for NSD3/MYC TR-FRET assay development. HEK293T cells were seeded into a 384-well plate and transfected with various tagged DNA constructs. After 48 h, TR-FRET assay was carried out directly in the plate as described in “Materials and Methods” section. A total of 48 combinations were tested as indicated in Table 1. S/B ratio was calculated for each condition. The data are presented as mean ± SD from triplicate samples. (B) The principle of our selected system for NSD3/MYC TR-FRET assay development. In cell lysate with overexpression proteins, VF-NSD3 protein was labeled with Tb through anti-Flag-Tb (N) to serve as a TR-FRET donor. GST-MYC (M) was coupled to TR-FRET acceptor d2 using anti-GST-d2. Interaction between NSD3 and MYC brings Tb and d2 into proximity, leading to the generation of TR-FRET signals. (C) TR-FRET signal from titration of stock cell lysate in a 384-well format. Transfection was performed in a six-well plate and stock cell lysate was collected after 48 h. Increasing amount of stock cell lysate with overexpressing VF-NSD3 and GST-MYC (15 μL) was mixed with diluted anti-Flag-Tb and anti-GST-d2 antibodies (15 μL). The TR-FRET signal was measured. The data shown are average from triplicate samples with SD. GST, glutathione S-transferase; NSD3, nuclear receptor binding SET domain protein 3; S/B, signal-to-background; SD, standard deviation; Tb, terbium; TR-FRET, time-resolved fluorescence resonance energy transfer; VF, Venus-Flag. Color images available online at www.liebertpub.com/adt