Fig. 1.
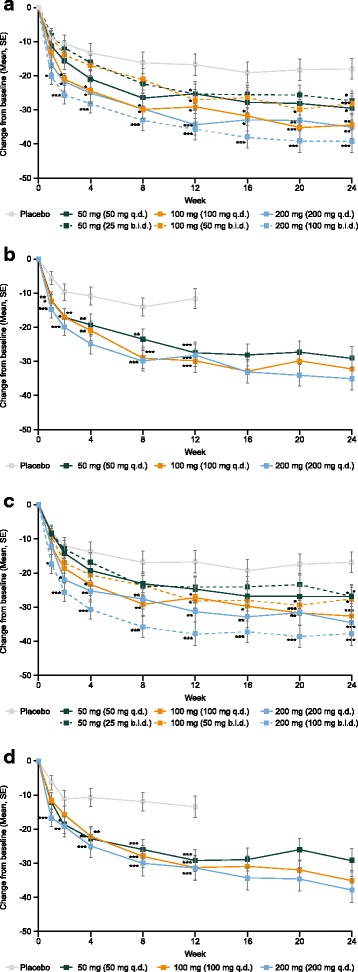
Patient Global scores, methotrexate (MTX) add-on (a) and monotherapy (b); Patient Pain scores, MTX add-on (c) and monotherapy (d). P values given for pair-wise comparison with placebo: *p < 0.05; **p < 0.01; ***p < 0.001. At week 12, patients receiving placebo in the MTX add-on study and patients receiving placebo and filgotinib 50 mg once daily (q.d.) in the monotherapy study, who had not achieved a 20% improvement in swollen joint count (SJC) and tender joint count (TJC) were reassigned to receive filgotinib 100 mg q.d. (both studies) or 50 mg twice daily (b.i.d.) (MTX add-on study). Patients who switched treatments at week 12 were handled as discontinuations and data were imputed from week 12 onwards using last observation carried forward. SE, standard error
