Fig. 2.
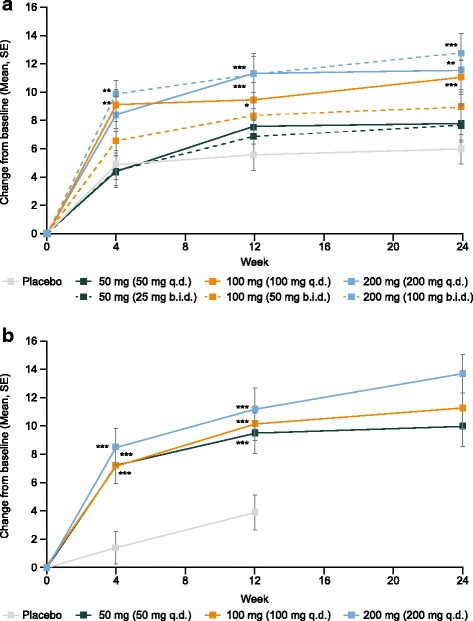
Functional Assessment of Chronic Illness Therapy (FACIT)-Fatigue, methotrexate (MTX) add-on (a) and monotherapy (b) studies. P values given for pair-wise comparison with placebo: *p < 0.05; **p < 0.01; ***p < 0.001. At week 12, patients receiving placebo in the MTX add-on study and patients receiving placebo and filgotinib 50 mg once daily (q.d.) in the monotherapy study who had not achieved a 20% improvement in swollen joint count (SJC) and tender joint count (TJC) were reassigned to receive filgotinib 100 mg q.d. (both studies) or 50 mg twice daily (b.i.d.) (MTX add-on study only). Patients who switched treatments at week 12 were handled as discontinuations and data were imputed from week 12 onwards using last observation carried forward. SE, standard error
