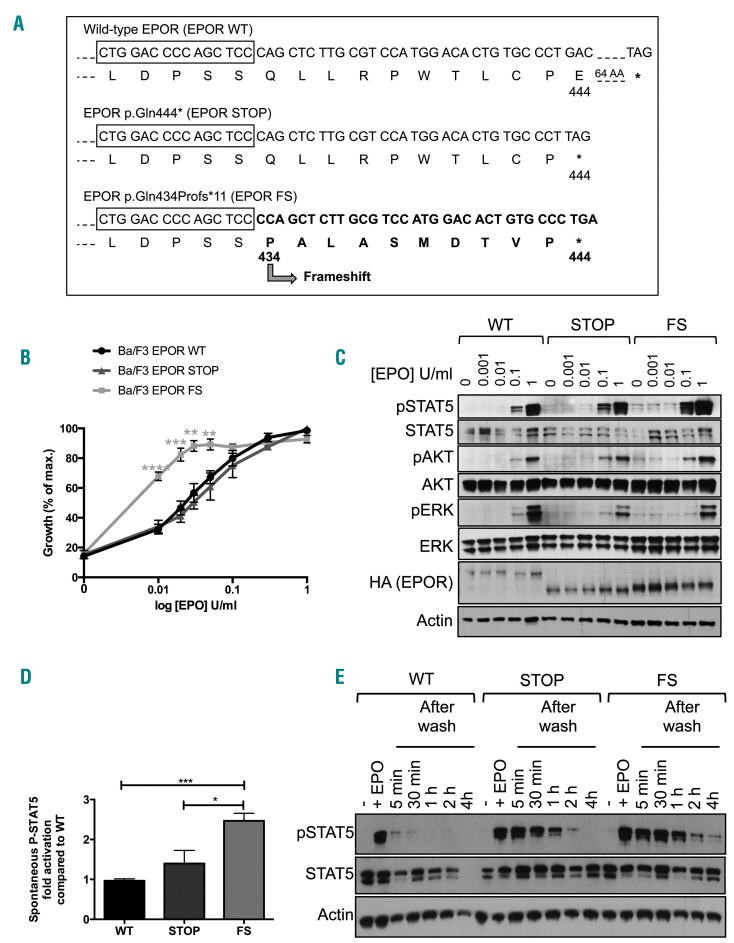
Figure 2.
Functional study of EPOR c.1300dup (p.Gln434Profs*11) in the Ba/F3 cell line. (A) Ba/F3 cells were transduced with pMX-HA-huEPOR-IRES-GFP retrovirus to stably express the wild-type receptor (EPOR WT), a truncated mutant at position 444 (p.Gln444*, EPOR STOP) or the frameshift mutant EPOR c.1300dup (p.Gln434Profs*11, EPOR FS). (B) Proliferation was assessed 48 h after culturing Ba/F3-EPOR cells in the absence or presence of increasing doses of erythropoietin (EPO) (0.01, 0.02, 0.03, 0.05, 0.1, 0.3, and 1 U/mL) by a WST-1 proliferation assay. Dose-response curves are means expressed in percentages of maximum growth value ± SEM (n = 3 in triplicate). Two-tailed t-test, *P<0.05, **P<0.01, ***P<0.001, ****P<0.0001. (C) Effect of EPO concentration on EPOR signaling. Ba/F3 cells expressing different EPOR constructs were examined by western blotting for the presence and phosphorylation status of various signaling molecules. Cells were serum- and cytokine-starved for 5 h prior to stimulation for 15 min with increasing doses of EPO (0, 0.001, 0.01, 0.1 and 1 U/mL). Expression of β-actin was used as a loading control. One of three independent experiments is presented. (D) Phosho-STAT5/actin spontaneous phosphorylation was quantified compared to WT using Image J. Results represent the mean ± SEM (n = 3). Two-tailed t-test, *P<0.05, ***P<0.001, (E) Persistence of STAT5 phosphorylation. Ba/F3-EPOR cells were serum- and cytokine-starved for 5 h prior to 15 min of stimulation with EPO 1 U/mL. Cells were then washed to remove the EPO and cultured in the absence of cytokine or serum. STAT5 phosphorylation was examined by western blotting in a time-dependent manner: after 5 h of starvation (−), after EPO stimulation (+EPO) and at different times after EPO removal (5 min, 30 min, 1 h, 2 h and 4 h). One out of two independent experiments is presented.