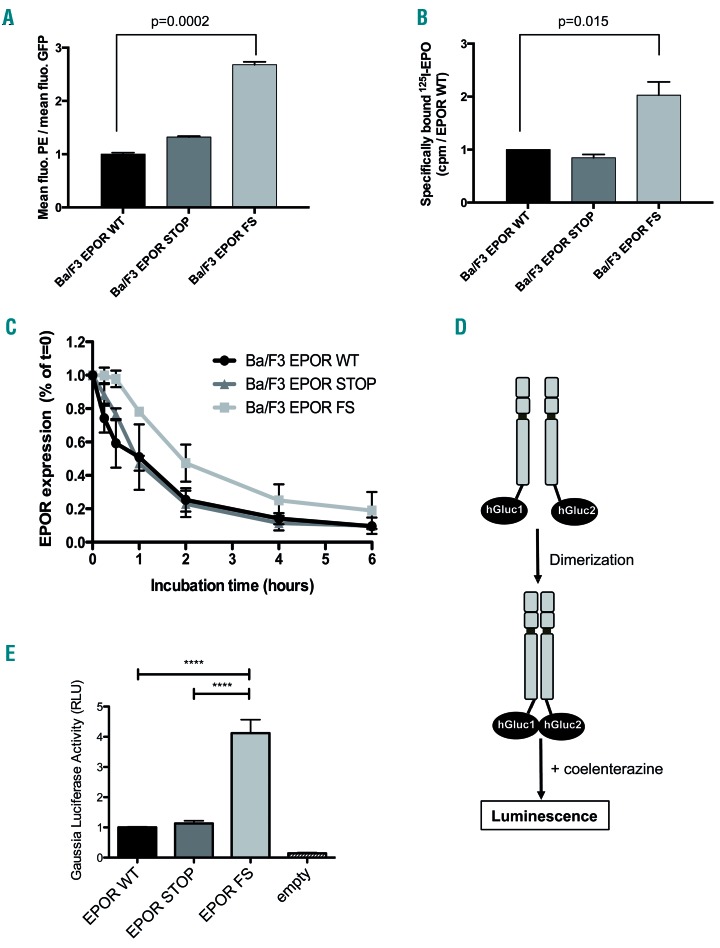
Figure 3.
Effects of c.1300dup (p.Gln434Profs*11) mutation on EPOR stability, dimerization and cell surface expression. (A) Cell-surface expression of the different EPOR was assessed by flow cytometry using PE fluorescent labeling of the extracellular HA-tag. The histogram shows the ratio of mean fluorescence intensiy (MFI) of PE-labeled cell-surface erythropoietin (EPO) on the respective MFI of GFP. Results are the mean ± SEM of seven independent experiments. (B) Cell-surface expression of the different EPOR was assessed with radiolabeled 125I-EPO. The results are expressed in cpm normalized to EPOR WT. The number of cell-surface receptors was determined by comparison between the radioactivity of transduced Ba/F3 cells and parental UT-7 cells that express 7000 receptors. (C) EPOR stability. Ba/F3-EPOR cells were incubated with cycloheximide for different times (0 min, 15 min, 30 min, 1 h, 2 h, 4 h, 6 h) and HA expression was studied by western blotting. HA-EPOR and β-actin were quantified using Image J software. The curves represent the HA/β-actin ratios. Three independent experiments were done. (D) Schematic representation of split Gaussia princeps luciferase complementation assay used to test EPOR dimerization in HEK293-derived BOSC cells. (E) Dimerization of human EPOR monomers was assessed by split Gaussia luciferase assay in steady-state conditions, in the absence of EPO in HEK cells. Close proximity between the C-terminal cytosolic domains of EPOR is significantly promoted by EPOR FS. The results represent the mean ± SEM from three independent experiments, each performed with eight biological replicates per condition. For each experiment, raw values were normalized to the average of the EPOR WT condition before pooling the data of all experiments together. Unpaired two-tailed t-test with Welch correction, ****P<0.0001.