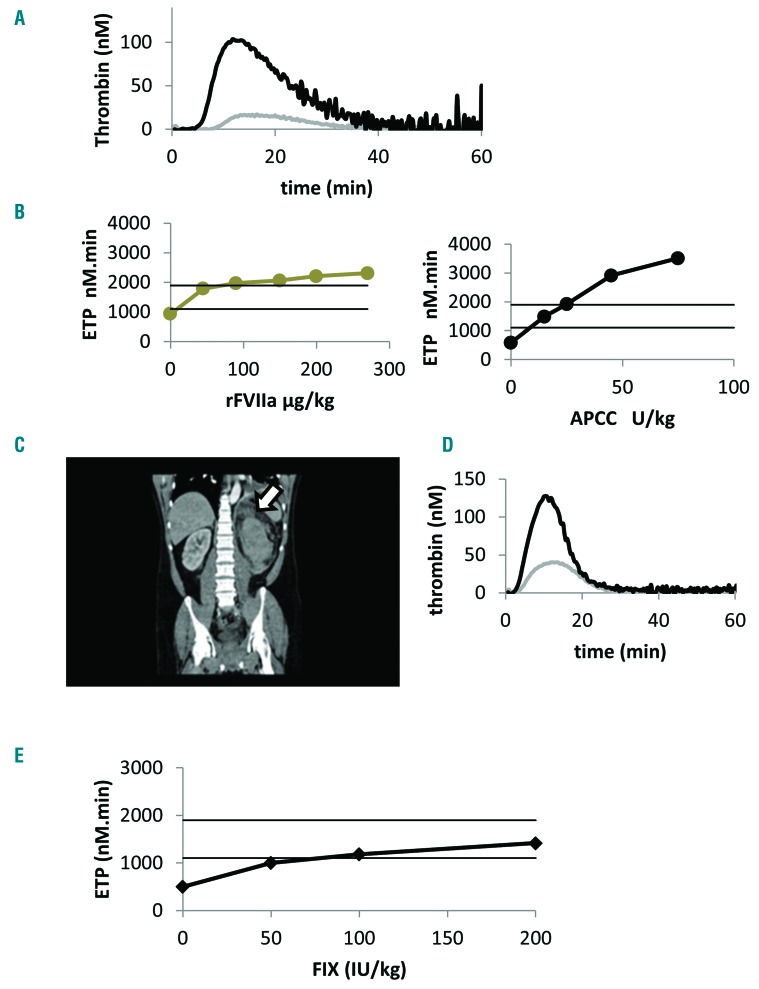
Figure 1.
Personalized management of breakthrough bleeds in a patient on prophylaxis with emicizumab. (A) Representative ex vivo thrombin generation curve obtained before prophylaxis with emicizumab showing a full normalization of ETP one hour after the infusion of APCC 75 U.kg−1. Results were obtained in platelet-poor plasma using a final concentration of tissue factor at 1pM and phospholipids at 4μM. (B) Representative thrombin generation results obtained before and after the in vitro addition of BPAs during emicizumab prophylaxis of 1.5 mg.kg−1 weekly. Two horizontal black lines represent the normal range of thrombin generation (ETP= 1487±372 nM.min; mean±2SD). It is noteworthy that the baseline thrombin generation capacity induced by emicizumab 1.5 mg.kg−1 was higher in platelet-rich plasma compared to platelet-poor plasma (ETP= 940 vs. 585 nM.min respectively). The efficacy of APCC was tested in platelet-poor plasma using a final concentration of tissue factor at 1pM and phospholipids at 4μM. rFVIIa was tested in platelet-rich plasma in the presence of TF 1pM. (C) Contrast-enhanced CT scan of the abdomen and pelvis, showing a left perirenal hematoma (arrow). (D) Thrombin generation curves obtained during prophylaxis with emicizumab, before initiating APCC (gray curve) and at day three of APCC treatment with 15 U.kg−1 showing a full normalization of ETP with no hypercoagulability. In vivo thrombin generation capacity induced by emicizumab prophylaxis (gray curve Figure 1D, ETP=600 nM.min) was higher than that observed in the absence of procoagulant treatment as represented in Figure 1A (gray curve ETP=115 nM.min). In addition, it was interesting to observe a prolonged action of APCC when combined with emicizumab. This might be explained by the mechanism of action and the prolonged half-life of emicizumab that might influence the half-life of its targets FIX and FX. Results were obtained in platelet-poor plasma using a final concentration of tissue factor at 1pM and phospholipids at 4μM. (E) Thrombin generation results obtained before and after the in vitro addition of rFIX (Benefix) during emicizumab prophylaxis of 1.5 mg.kg−1 weekly. The efficacy of rFIX 0-25-50-100 IU.kg−1 was tested in platelet-poor plasma using a final concentration of tissue factor at 1pM and phospholipids at 4μM. ETP: endogenous thrombin potential; APCC: activated prothrombin complex concentrate: rFVIIa: recombinant activated factor VII; FIX: factor IX.