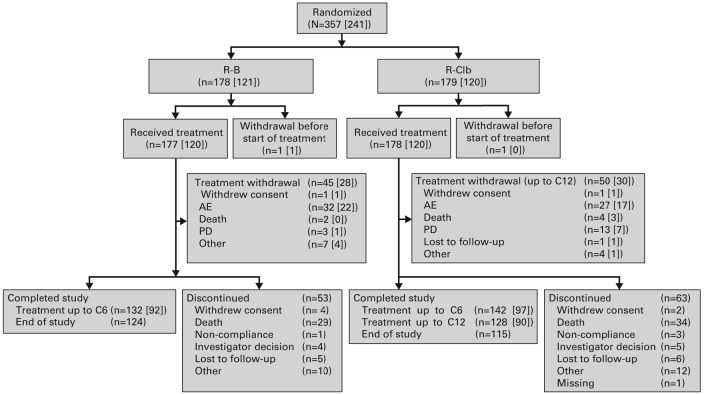
Figure 1.
Patient disposition. Numbers in parentheses represent the number of patients from the 1L subpopulation. In total, 118 patients (33%) discontinued the study prematurely, due to death (R-B 16%; R-Clb 19%), patient lost to follow-up (3% per arm), investigator decision (R-B 2%; R-Clb 3%), patient withdrew consent (R-B 3%; R-Clb 2%), patient non-compliance (R-B 1%; R-Clb 2%), and ‘other’ reasons (R-B 6%; R-Clb 7%). Reason for withdrawal was not available for one patient (R-Clb). AE: adverse event; C: cycle; N: number of patients; PD: progressive disease; R-B: rituximab plus bendamustine; R-Clb: rituximab plus chlorambucil.