Abstract
In October 2017, the International Olympic Committee hosted an international expert group of physical therapists and orthopaedic surgeons who specialize in treating and researching pediatric anterior cruciate ligament (ACL) injuries. The purpose of this meeting was to provide a comprehensive, evidence-informed summary to support the clinician and help children with ACL injury and their parents/guardians make the best possible decisions. Representatives from the following societies attended: American Orthopaedic Society for Sports Medicine; European Paediatric Orthopaedic Society; European Society for Sports Traumatology, Knee Surgery, and Arthroscopy; International Society of Arthroscopy, Knee Surgery and Orthopaedic Sports Medicine; Pediatric Orthopaedic Society of North America; and Sociedad Latinoamericana de Artroscopia, Rodilla, y Deporte. Physical therapists and orthopaedic surgeons with clinical and research experience in the field and an ethics expert with substantial experience in the area of sports injuries also participated. This consensus statement addresses 6 fundamental clinical questions regarding the prevention, diagnosis, and management of pediatric ACL injuries. Injury management is challenging in the current landscape of clinical uncertainty and limited scientific knowledge. Injury management decisions also occur against the backdrop of the complexity of shared decision making with children and the potential long-term ramifications of the injury.
Keywords: pediatric, injury prevention, knee, child, evidence based practice
The number of anterior cruciate ligament (ACL) injuries in children is rising.112,133 ACL injuries in children create a level of concern that is more significant than in any other population with ACL injury. Do children who rupture their ACL mature similarly to their uninjured peers? Do they continue with sport? Do they prioritize their education and other interests over sport? Does an ACL injury and treatment change their lives? These young individuals have to live with their knee problem for the rest of their lives, which may compromise their quality of life and increase the risk for further injury, meniscal tears, and early-onset osteoarthritis.134 Compounding the problem is that there is very little high-quality evidence to guide decision making in the management of pediatric ACL injuries.91
Progress on these issues can be made only on the basis of long-term follow-up in multicenter collaborations. Achieving progress requires a long-term commitment from those who have children’s interests close at heart. Therefore, in October 2017, the International Olympic Committee (IOC) hosted an international expert group of physical therapists and orthopaedic surgeons who specialize in treating and researching pediatric ACL injuries. Representatives from the following societies attended: American Orthopaedic Society for Sports Medicine (AOSSM); European Paediatric Orthopaedic Society; European Society for Sports Traumatology, Knee Surgery, and Arthroscopy (ESSKA); International Society of Arthroscopy, Knee Surgery and Orthopaedic Sports Medicine (ISAKOS); Pediatric Orthopaedic Society of North America; and Sociedad Latinoamericana de Artroscopia, Rodilla, y Deporte (SLARD).
Clinicians are charged with the responsibility of providing accurate information and effective treatment to this vulnerable population. Sharing information about the potential consequences of ACL injury and treatment in childhood to long-term knee health should be a central part of the shared decision-making process. Adult patients with ACL injury may develop symptoms and signs of osteoarthritis within 10 years of the index injury.60 Therefore, the clinical concern is that a child who is injured at the age of 10 years could have symptomatic osteoarthritis by the age of 20 years. A quintessential question is, therefore, What is the long-term prognosis after ACL injury in childhood? Having a definitive, evidence-based answer to this question will strengthen our confidence in clinical decision making. Clearly, the answer to this question is not straightforward and depends on many factors, but an important point is that long-term outcomes after ACL injury in childhood, including the development of osteoarthritis, have not been studied.
Injury management is challenging in the current landscape of clinical uncertainty and limited scientific knowledge. Injury management decisions also occur against the backdrop of the complexity of shared decision making with children and the potential long-term ramifications of the injury. This consensus statement addresses 6 fundamental clinical questions regarding the prevention, diagnosis, and management of pediatric ACL injuries (Table 1). By framing each topic around clinical questions, the aim of this consensus statement was to provide a comprehensive, evidence-informed summary to support the clinician and help children with ACL injury and their parents/guardians make the best possible decisions.
TABLE 1.
Fundamental Clinical Questions and Relevant Consensus Statement Topicsa
| Section: Question | Relevant Consensus Statement Topics |
|---|---|
| 1: How can the clinician prevent ACL injuries in children? | Injury prevention |
| 2: How does the clinician diagnose ACL injuries in children? | Diagnosis, clinical tests, and imaging |
| 3: What are the treatment options for the child with an ACL injury? | High-quality rehabilitation Surgical techniques The pediatric ACL graft |
| 4: What are the most important considerations when making treatment decisions? | Skeletal age assessment The decision for ACL reconstruction Risks associated with ACL reconstruction Management of associated injuries |
| 5: How does the clinician measure outcomes that are relevant to the child with an ACL injury? | Pediatric patient-reported outcome measures |
| 6: What are the clinician’s roles and responsibilities? | Ethical considerations |
aACL, anterior cruciate ligament.
Consensus Methods
A modified Delphi consensus process32,37,127 was used to identify the topics to be addressed in this consensus statement. Experts were contacted by email in June 2016 and invited to respond to an electronic survey. A mix of open and closed questions was used to gather expert opinion regarding the key issues in the field. These responses were summarized and formed the basis of 18 statements regarding injury prevention, diagnosis, prognosis, surgical techniques, treatment decision making, management, and outcome measurement (see Appendix Table A1).
A 2-round consensus process was conducted, involving 19 content experts. Respondents rated the importance of the 18 predefined statements on an 11-point scale ranging from “not important at all” to “of utmost importance.” Consensus was defined as a mean ranking of at least 8 points for each statement. After the first voting round, statements reaching consensus were removed so that only statements that failed to reach consensus went through to the second voting round. The statements that finally reached consensus formed the topics that were discussed at the consensus meeting.
The IOC convened a consensus meeting of 21 experts in Lausanne, Switzerland, in October 2017. The experts were identified by the IOC through the AOSSM, ESSKA, ISAKOS, and SLARD member societies and from physical therapists and orthopaedic surgeons with clinical and research experience in the field. An ethics expert with substantial experience in the area of sports injuries also participated.
Section 1: Injury Prevention
This section addresses the fundamental clinical question, How can the clinician prevent ACL injuries in children? Prevention of ACL injury is important because of the potential for serious long-term consequences in those who sustain the injury and because of the increased risk of reinjury to either knee.100 Therefore, it is paramount that the principles of injury prevention be incorporated in the treatment of the child with an ACL injury.
Substantial advances have been made in the development and application of ACL injury prevention programs across numerous pivoting sports. There is compelling evidence that ACL injury prevention programs work in skeletally mature patients: they reduce the number of athletes who sustain a primary ACL injury and lower the number of new ACL injuries among athletes who return to sport after primary ACL injury.86,95,115,117,120,130
The athlete’s biomechanical movement patterns are a key modifiable risk factor for injury. Injury prevention programs target movement patterns by incorporating strength, plyometrics, and sports-specific agility training.36,80 Coach and athlete education on cutting/landing techniques (eg, wide foot position when cutting, flexed knee when landing) that avoid high-risk knee positions is also fundamental. Injury prevention programs are straightforward to implement because they require little to no equipment and are performed as part of regular team training or physical education 2 to 3 times per week (Figure 1).
Figure 1.
Injury prevention exercises incorporated into team training.
FIFA 11+ for Kids
Injury prevention programs should also be implemented early in the athlete’s developmental process. This will give the athlete the best opportunity to develop strong and favorable movement strategies. One well-established injury prevention program,126 the Fédération Internationale de Football Association (FIFA) 11+, was recently modified to suit the pediatric population: FIFA 11+ for Kids (eg, adding falling techniques, making partner-based exercises more play oriented). Completing the program can reduce football-related lower extremity injuries by over half.107 Children who complete the program also have improved motor control, balance tests, and agility as compared with those who do not complete the program.106
Factors That Might Affect Injury Prevention Effectiveness
Well-designed injury prevention programs have the lowest injury rates and injury time loss,12,126 but the effect of a well-designed injury prevention program is strongly influenced by how frequently athletes perform the training.52,118,119 Therefore, consistent implementation, utilization, and adherence across all levels of competitive play are one of the biggest challenges facing the clinician. Those involved in youth sports and clinicians who treat pediatric athletes with ACL injury have a responsibility to actively advocate for injury prevention in a primary setting and for children who return to sport after an injury.
Section 2: Diagnosis, Clinical Tests, and Imaging
This section addresses the fundamental clinical question, How does the clinician diagnose ACL injuries in children? High-quality injury prevention programs are the first-line defense against the potential negative short- and long-term consequences of ACL injury. However, if injury prevention efforts fail, timely and accurate diagnosis is important, since diagnosis is the starting point for effective management planning and shared decision making. The clinician combines information from the patient’s history, examination, clinical tests, and imaging to build the clinical picture that will inform diagnosis and treatment. Typically, a thorough history and clinical examination will enable the clinician to make an accurate diagnosis.
Clinical Pearl 1: Hemarthrosis (acute swelling in the knee within 24 hours after a trauma, attributed to intra-articular bleeding) following acute knee injury is an important clue suggesting structural knee injury.
Clinical Pearl 2: Diagnosis can be more challenging in children than adults because children may be poor historians and have greater physiologic joint laxity (be sure to examine both knees) and because magnetic resonance imaging (MRI) interpretation is more difficult given developmental variants in children.62,124
Clinical Pearl 3: Because of the immature skeleton, children may sustain different knee injuries than adults (eg, sleeve fracture of the patella, epiphysiolysis).
Consider starting the assessment by ordering plain knee radiographs for all pediatric patients with a hemarthrosis or suspected acute knee injury. The reason is that tibial eminence fractures and an ACL tear can present with similar histories and physical examination findings. It is also important to rule out other pediatric fractures (eg, epiphyseal fracture, sleeve fracture of the patella). Perform an MRI to confirm the diagnosis of ACL injury and evaluate other soft tissue structures.65 In children with an ACL injury, MRI may yield additional information to identify meniscal tears, other ligament injury, or osteochondral injury. In children with a locked knee, acute MRI is warranted to assess the presence of a displaced bucket-handle meniscal tear or an osteochondral injury that may need prompt surgical treatment.
Measurement Properties for Clinical Examination and MRI
No single question, test, or image can accurately identify an ACL injury every time. The measurement tools available to the clinician are not perfect, but they do yield valuable information in the clinical context. Knowledge of the measurement properties of clinical tools helps the clinician balance the information gained from these tools. The negative predictive values of clinical examination and MRI for ACL tear and meniscal pathology are greater than the positive predictive values (Table 2). This means that if clinical examination or MRI is negative for injury, the chance of the patient's having an injury is low. However, if the tests are positive, it does not mean that the clinician can always reliably rule the diagnosis in.
TABLE 2.
Diagnostic Accuracy of Clinical Examination and MRI in Intra-articular Knee Disordersa
| Sensitivity, % | Specificity, % | Positive Predictive Value, % | Negative Predictive Value, % | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Diagnosis | Clinical | MRI | P Value | Clinical | MRI | P Value | Clinical | MRI | Clinical | MRI |
| ACL tear | 81.3 | 75.0 | .55 | 90.6 | 94.1 | .39 | 49.0 | 58.6 | 97.8 | 97.1 |
| Medial meniscus tear | 62.1 | 79.3 | .15 | 80.7 | 92.0 | .03b | 14.5 | 34.3 | 97.6 | 98.8 |
| Lateral meniscus tear | 50.0 | 66.7 | .24 | 89.2 | 82.8 | .21 | 34.0 | 30.1 | 94.1 | 95.7 |
aAdapted from Kocher et al.65 Clinical examination included patient history, physical examination, and radiographs performed by a pediatric orthopaedic sports medicine specialist or a postresidency pediatric sports medicine fellow. ACL, anterior cruciate ligament; MRI, magnetic resonance imaging.
bP < .05.
Section 3: Treatment of ACL Injuries in Children
This section addresses the fundamental clinical question, What are the treatment options for the child with ACL injury? Once the clinician is certain of the injury diagnosis, he or she first needs to know the available treatment options and discuss these options with the child and the child’s parents/guardians so that a shared decision can be made about how best to manage the knee injury.
The goals of treatment for the child with ACL injury are as follows:
To restore a stable, well-functioning knee that enables a healthy active lifestyle across the life span
To reduce the impact of existing, or the risk of further, meniscal or chondral pathology, degenerative joint changes, and need for future surgical intervention
To minimize the risk of growth arrest and femur and tibia deformity
Two treatment options can help the child with an ACL injury (with or without associated knee injuries) achieve these goals: high-quality rehabilitation alone (nonsurgical treatment) and ACL reconstruction plus high-quality rehabilitation. This section describes the key components of high-quality rehabilitation for the child with an ACL injury and the options for the ACL reconstruction surgical technique. Section 4 outlines potential treatment decision modifiers.
High-Quality Rehabilitation
High-quality rehabilitation is a critical component in the management of ACL injury, and the principles of rehabilitation are the same, irrespective of whether the child has had an ACL reconstruction or has elected for nonsurgical treatment. Guidance for pediatric rehabilitation is extrapolated from clinical experience and research in adults, although it is uncertain whether adult principles apply to children.138 Pediatric rehabilitation must be performed in close collaboration with the child’s parents/guardians. Exercises and functional goals must be modified and not simply copied from the adult-oriented rehabilitation protocols that may be more familiar to many clinicians. The reason is that children are not small adults—they cannot be expected to perform unsupervised training independently, with perfect technique. Qualified rehabilitation clinicians must supervise rehabilitation for the child with an ACL injury.
Rehabilitation Focus
Dynamic, multijoint neuromuscular control is the primary focus of ACL rehabilitation in children. For the youngest patients (with markedly open physes, age <12 years), there is less emphasis on the development of muscular strength and hypertrophy. During maturation and throughout the onset of puberty, rehabilitation strategies that more closely resemble those used with adult patients are appropriate given the increase in androgenic hormones.15 These strategies must include heavier and externally loaded strength training.
Rehabilitation must be thorough and individualized to the child’s physiologic and psychological maturity to achieve successful outcomes: emphasize exercises that facilitate dynamic lower limb alignment and biomechanically sound movement patterns. Although this has been successfully implemented in the rehabilitation programs of adolescents and adults, it has not yet been documented as extensively in children. The exercises are gradually progressed through phases 2 and 3 of the pediatric ACL rehabilitation protocol (Table 3 and Appendix Table A2) as part of sport-specific rehabilitation. Appendix Table A2 provides examples of exercises to consider in each rehabilitation phase. Reinjury anxiety and the patient’s confidence in his or her injured knee affect outcomes after ACL rehabilitation among adults.8,9 These psychological factors are also likely to be important in the pediatric population but currently are insufficiently studied.
TABLE 3.
Recommended Functional Tests and Return-to-Sport Criteria for the Child and Adolescent With ACL Injurya
| For patients who choose ACL reconstruction | |
| Prehabilitation |
|
| For patients who choose ACL reconstruction OR nonsurgical treatment | |
| Phase 1 to phase 2 |
|
| Phase 2 to phase 3 |
|
| Phase 3 to phase 4: sport participation (return-to-sport criteria) and continued injury prevention |
|
aMuscle strength testing should be performed with isokinetic dynamometry or handheld dynamometry/1-repetition maximum. The type of test and experience of the tester are highly likely to influence the results. If using handheld dynamometry/1-repetition maximum, consider increasing the limb symmetry criterion cutoff by 10% (ie, 90% limb symmetry becomes 100% limb symmetry). Clinicians who do not have access to appropriate strength assessment equipment should consider referring the patient elsewhere for strength evaluation. ACL, anterior cruciate ligament.
Following surgical treatment, the graft type used for ACL reconstruction and associated injury or surgery to other ligaments, menisci, or articular cartilage necessitates specific adjustments to the rehabilitation program. Rehabilitation programs should be designed to allow the child to participate in his or her team practice sessions to maintain the social benefits of staying within the team. Parents or guardians should be active participants in the daily rehabilitation.101 This may include assisting the child in technical and functional exercises during team practice (eg, short passes in soccer).
Rehabilitation Phases
Rehabilitation for the child with an ACL injury is organized into 4 phases, with an additional prehabilitation phase for those who choose ACL reconstruction (Table 3 and Appendix Table A2). Specific clinical and functional milestones should be met before progressing from one phase to the next.128 Throughout the first 2 phases, the child should be guarded from cutting and pivoting activities during sport, free play, and physical education classes in school.
Rehabilitation Progression
The framework for progression through functional milestones is similar for ACL reconstruction and nonsurgical treatment. However, there are different expectations for progression and time to return to full participation in sport. For all patients, rehabilitation progression must be guided by clinical and functional milestones, and return to full participation7 is dependent on successfully achieving the return-to-sport criteria (Table 3). Nonsurgical treatment should last for at least 3 to 6 months.49 Postoperative rehabilitation should last for a minimum of 9 months before return to full participation in preferred physical activities.50
Data from international registries suggest that young athletes are at high risk for a second ACL injury following an ACL reconstruction,76 and the risk is greatest in the first 12 months postoperatively.28,50 Therefore, consider advising the child athlete not to return to pivoting sports until at least 12 months following ACL reconstruction. Rehabilitation is also an excellent opportunity to train the uninjured leg, which might be important considering the risk of contralateral injury.28 Once the child returns to sport, a comprehensive injury prevention program emphasizing biomechanical alignment and landing/cutting technique should be integrated with usual training.
Considerations When Designing Rehabilitation Programs for the Prepubescent Child
Children who are close to skeletal maturity may follow rehabilitation128 and return-to-sports guidelines50,77 intended for adults. There are 5 important considerations for the prepubescent child:
Consider a home-based program with emphasis on playful exercises and variation (Figure 3) to discourage boredom.
Single-leg hop tests and isokinetic strength tests have larger measurement errors in the prepubescent population, so use these tests with caution.59
Focus on evaluating the quality of movements during single-leg hop testing instead of the leg symmetry index measures.
Tests and criteria to assess movement quality are yet to be validated, so the responsible clinician needs to have skills and experience in this area.
Return-to-sport criteria were designed and scientifically tested in the skeletally mature patient and are recommended for the child who is close to maturity.50,125 The validity of these criteria in the prepubescent child is unknown.
Figure 3.
One example of an exercise that could be incorporated into a home-based anterior cruciate ligament rehabilitation program.
Figure 2.
Child demonstrating how to hold terminal knee extension during single-limb stance. This is an important marker of quadriceps control in anterior cruciate ligament rehabilitation and prehabilitation.
Bracing
Many clinicians involved in nonsurgical treatment of skeletally immature children recommend that the child wears a protective brace during strenuous physical activities.93 The child who has had surgical treatment typically wears a brace during the prehabilitation phase until ACL reconstruction is performed. Following surgery, it is recommended that the child wear a protective knee brace through the successful completion of the functional milestones in rehabilitation phase 1 (usually 2 to 6 weeks postoperatively, depending on concomitant surgical procedures). However, the effectiveness of bracing following ACL injuries or reconstruction in pediatric patients is unknown. Other considerations related to the use of a brace might be to prevent knee hyperextension or knee valgus/varus, to enhance the child’s awareness of his or her injury, and as a protective signal to others whom the child might encounter (eg, at school).
Surgical Techniques
The general principles of ACL reconstruction in adults apply to the pediatric patient: use a well-positioned (soft tissue) autograft of adequate size, with adequate fixation to allow functional rehabilitation. Physeal damage should be minimized to avoid growth disturbance. Bone plugs and fixation devices should not cross the physis.41,68,111
Key Indications for ACL Reconstruction
There are 3 indications for pediatric ACL reconstruction:
The child has repairable associated injuries that require surgery (eg, bucket-handle meniscus tear, repairable meniscal lesion, or osteochondral defect).
The child has recurrent symptomatic knee giving way after completing high-quality rehabilitation.
The child experiences unacceptable participation restrictions (ie, an unacceptable modification of activity level to avoid knee giving way).
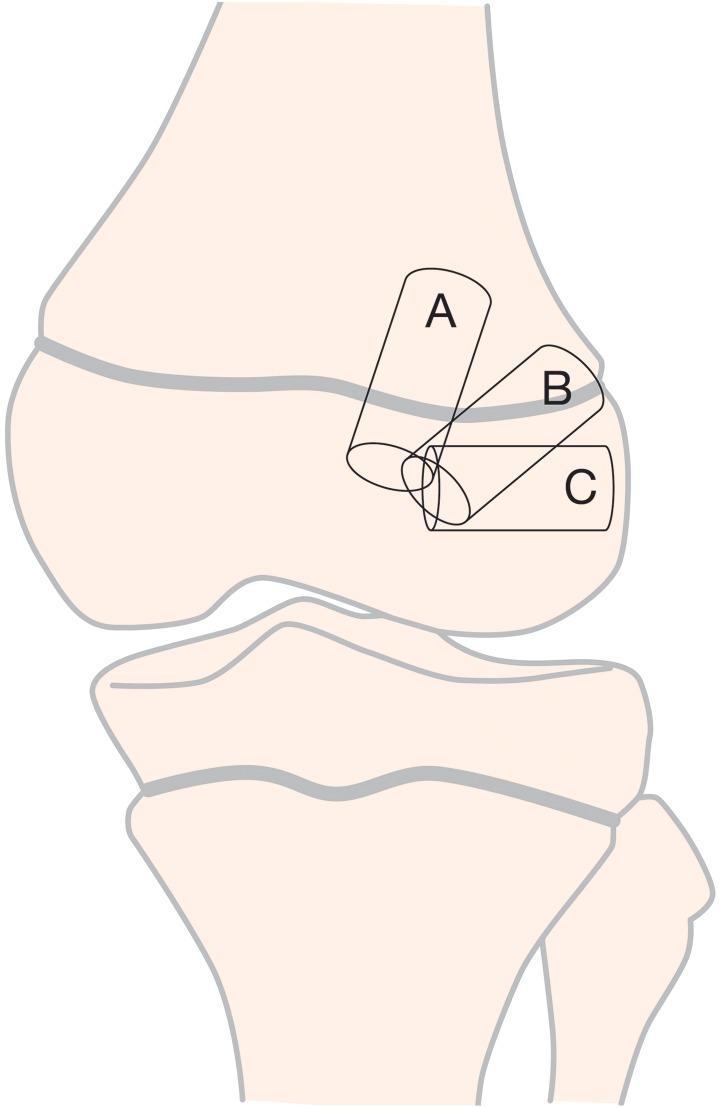
ACL Reconstruction Techniques
There are 3 possible techniques for pediatric ACL reconstruction.
Transphyseal ACL Reconstruction
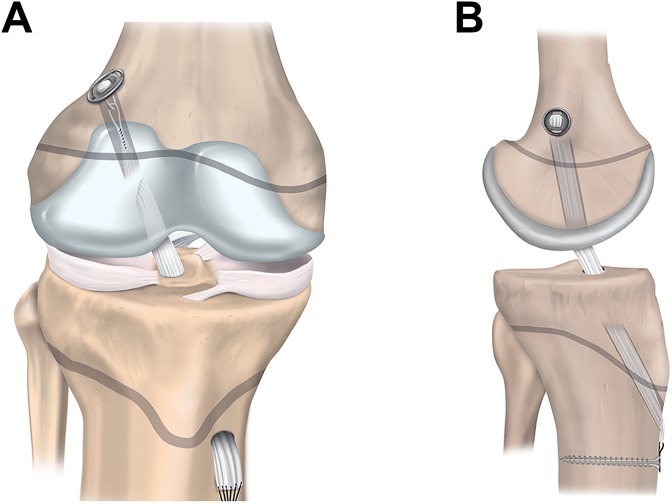
The transphyseal technique in the child is similar to the technique that the surgeon would use for ACL reconstruction in adults. Single-bundle transphyseal ACL reconstruction with a quadrupled hamstring graft is the most common (Figure 4).21,25,38,55,71,114 Therefore, because the surgeon is more likely to be familiar with the key elements of the procedure, it may reduce the risk of intraoperative complications. Ensure that the diameter of the bone tunnels is as small as possible (<9 mm) to accommodate an appropriately sized graft.58 Similarly, to minimize physeal damage, orient the tibial tunnel as vertically and centrally as possible while maintaining the anatomic position of the graft. On the femoral side, the surgeon should take care to avoid the perichondral ring. Drilling via the anteromedial portal can result in a tunnel that has an elliptical trajectory through the physis. Consider a slightly more vertical orientation than what might be used for an ACL reconstruction in an adult patient, or choose a different drilling approach.
Figure 4.
Transphyseal anterior cruciate ligament reconstruction: (A) anterior and (B) lateral views.
Physeal-Sparing ACL Reconstruction
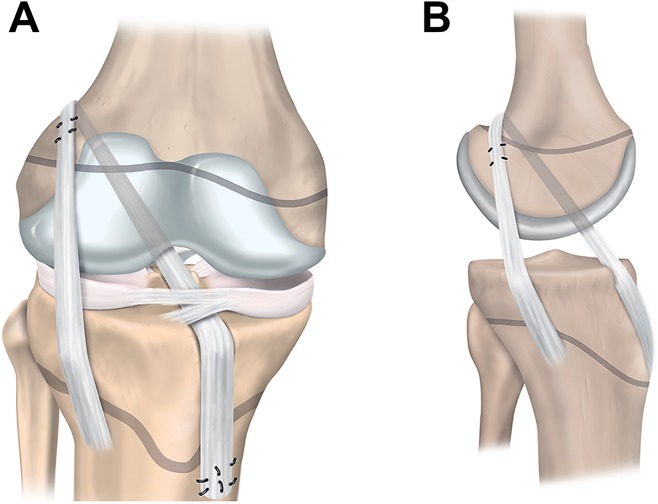
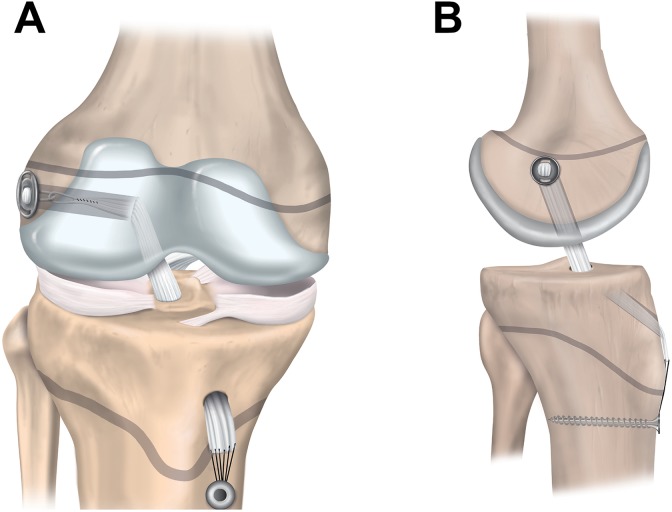
Physeal-sparing techniques avoid physeal damage in patients with markedly open physes. The techniques include an over-the-top technique with a strip of the iliotibial band (Figure 5)67 and an all-epiphyseal procedure (Figure 6).3 In the all-epiphyseal procedures, use of fluoroscopic visualization is recommended to reduce the risk of physeal damage. With the over-the-top technique, avoid femoral rasping to minimize the risk for damage to the perichondral ring.
Figure 5.
Physeal-sparing anterior cruciate ligament reconstruction with an over-the-top technique and iliotibial band: (A) anterior and (B) lateral views.
Figure 6.
Physeal-sparing anterior cruciate ligament reconstruction with an all-epiphyseal technique: (A) anterior and (B) lateral views.
Partial Transphyseal ACL Reconstruction
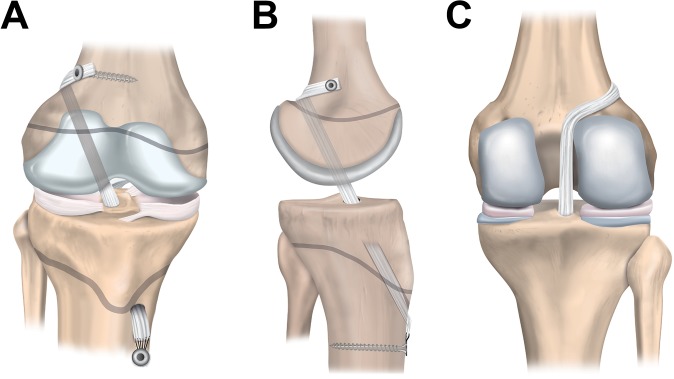
The partial transphyseal technique (Figure 7) combines a transphyseal tibial tunnel with a physeal-sparing technique on the femoral side.5,53,82
Figure 7.
Partial transphyseal anterior cruciate ligament reconstruction: (A) anterior, (B) lateral, and (C) posterior views.
Surgical Principles and Techniques for Growth Disturbance Risk Reduction
Drill-hole trajectory and location influence the degree of risk to the physes (Table 4 and Figure 8). Knowledge of 3 key principles will help the surgeon minimize the risk to the physes during transphyseal ACL reconstruction:
TABLE 4.
Three Options for Femoral Tunnel Trajectoriesa
| Tunnel Option | Considerations |
|---|---|
| A: Vertical transphyseal | |
| Advantage | Minimizes physeal volume affected |
| Disadvantage | Less-than-ideal coverage of ACL footprint |
| B: Oblique transphyseal | |
| Advantage | Anatomic graft position covering the ACL footprint |
| Disadvantage | Greater volume of physis negatively affected |
| C: Horizontal all epiphyseal | |
| Advantage | Appropriate placement at ACL footprint; no drilling through the physis |
| Disadvantage | Requires precise tunnel placement to reduce the risk for physeal damage |
aACL, anterior cruciate ligament.
Figure 8.
Three options for femoral tunnel trajectories: A, vertical transphyseal; B, oblique transphyseal; and C, horizontal all-epiphyseal.
Drilling at the periphery of the physis and the perichondral ring increases the risk of growth disturbance. Drill holes may be placed in an all-epiphyseal manner to allow for drilling at the native ACL footprint while avoiding the physis. Precise tunnel placement is required when performing this technique to avoid damage to the undulating distal femoral physis.
Bone tunnel drill holes should be as vertical as possible (while still maintaining anatomic graft position) and as central as possible. This is especially important when drilling through the anteromedial portal. Drilling an oblique tunnel rather than a more vertical tunnel increases the amount of physis removed and increases the risk for growth disturbance.
Do not cross the epiphysis with hardware, implants, or bone blocks. Fill bone tunnels with soft tissue rather than leaving the tunnels open.
Graft Choice and Fixation
Only soft tissue grafts (not allografts) should be used for ACL reconstruction in pediatric patients with open physes. The quadrupled hamstring graft is most common.25,38,55,71,114 A quadriceps tendon graft may be used.53 The patellar tendon should not be harvested in pediatric patients with open physes, to avoid damage to the tibial tubercle apophysis. Allografts are not indicated in pediatric patients in most cases, since the use of allografts in pediatric ACL reconstruction has shown poor clinical outcomes.61,108,123 A novel technique involving the use of living-donor hamstring tendon allograft was reported47,55 to avoid the varied sterilization techniques used in cadaveric soft tissue allografts and to preserve the neuromuscular unit of the growing patient.139,140 However, long-term clinical outcomes are yet to be assessed.
Extracortical fixation of soft tissue grafts may be performed with a cortical button, suture, post, or staple. Aperture fixation may be performed with interference screws, provided that the screws do not cross the physis.
Graft Incorporation
Data regarding ACL graft incorporation in children are scarce. Pediatric soft tissues have a greater biological growth potential as compared with adults,40,94 and cell migration and proliferation of ACL fibroblasts slow as a person grows older.83 The clinical relevance of the growth potential to pediatric ACL reconstruction is still unclear,102 although there is rationale from animal models that the pediatric ACL graft may remodel faster than the adult ACL graft.89
Adaptations and Remodeling in the Growing Child
The ACL graft must adapt as the child grows. The graft may increase in length as the bone grows, and the bone tunnels may reduce in relative size.16,73 It is uncertain whether the diameter of the intra-articular part of the graft becomes longer and thinner11 or not16 as the child grows. The graft does not increase in diameter as the child grows but may increase in length.10
The graft may become more vertically oriented with longitudinal bone growth after transphyseal ACL reconstruction. This observation might be explained by the movement of the femoral fixation site with physeal growth or because the tibial tunnel aperture becomes relatively more posterior owing to greater anterior growth of the proximal tibia. Other changes occurring as the child grows are secondary intercondylar notch narrowing, distal migration of the tibial and/or proximal migration of the femoral extracortical fixations, and verticalization of the Blumensaat line.110 However, the long-term clinical significance of these growth-related changes is unclear.
Section 4: Treatment Decision Modifiers
This section addresses the fundamental clinical question, What are the most important considerations when making treatment decisions? The key issues addressed relate to assessment of skeletal maturity, the decision for surgery, the management of injuries to other knee structures, and the potential adverse events following treatment. These issues may alter the ACL injury management decision depending on the risk tolerance of the decision-making team (which should include clinicians, the child, and the child’s parent/guardian).
Skeletal Age Assessment
Assessing and documenting the child’s skeletal age, in addition to his or her chronological age, is necessary for individualizing treatment of ACL injuries. The main goal with respect to skeletal age assessment is to define remaining knee growth. Protecting the physis and perichondral ring from damage during ACL reconstruction is an important consideration111; insult to a growth area that is near completion of growth can result in premature closure.
Estimating skeletal age and remaining growth are key considerations for treatment decision making. These estimates will guide choice of treatment, timing of surgery, and surgical method. Open physes in the child are vulnerable at surgery, and none of the current recommended surgical treatments for the child with an ACL injury can be guaranteed to protect the physis and avoid the potential complication of growth arrest or deformity (Table 5). The clinician might also consider long-leg radiographs (hips to ankles) after injury to establish a baseline for assessing the potential development of angular deformity and leg-length discrepancy. Assessing skeletal age is also relevant in research and may be beneficial for medicolegal reasons. If overgrowth, growth arrest, or deformity occurs, presurgical documentation of skeletal age may be important.
TABLE 5.
Considerations for Skeletal Age Assessment
|
Treating the Child With ACL Injury: To Operate or Not to Operate?
Children who have repairable additional injuries at ACL injury diagnosis (eg, displaced bucket-handle meniscal tear) should be treated with early ACL reconstruction and meniscal repair.75 For those without additional injuries warranting surgery, there are conflicting opinions regarding the best treatment approach. These approaches range from early ACL reconstruction for all children to primary nonsurgical management (high-quality rehabilitation alone) with the option of late ACL reconstruction (1) if the child has recurrent instability problems despite high-quality rehabilitation or (2) if he or she sustains secondary intra-articular injuries.
A well-performed ACL reconstruction and preservation of the meniscus can restore knee stability.66 However, if the child receives inadequate (or no) rehabilitation, the chances of recovering high-level function to safely participate in all aspects of life (including pivoting sports), for the rest of his or her life, might be slim. Similarly, high-quality rehabilitation will not salvage poor surgical treatment (eg, graft malposition).
Children who undergo ACL reconstruction after failed nonsurgical management may have a greater number of meniscal and chondral injuries at the time of ACL reconstruction as compared with those who undergo early ACL reconstruction.4,81,97 The number of instability episodes prior to surgery appears to be a more important factor than the length of time between injury and surgery.42 This consideration is the background for early surgery decisions. However, there is a lack of high-quality prospective studies investigating the outcomes of surgical and nonsurgical treatment for pediatric ACL tears.91
Nonsurgical treatment is a viable and safe treatment option for skeletally immature patients who do not have associated injuries or major instability problems.90 High-quality rehabilitation alone may stabilize the knee dynamically without compromising the physes and is a focused training program supervised by a qualified rehabilitation clinician (see section 3 for the key principles of high-quality rehabilitation). Nonsurgical treatment can be (1) a permanent treatment option for those who do not develop functional instability or (2) a short-term option to delay ACL reconstruction until the child has reached skeletal maturity. Abandoning nonsurgical treatment in favor of ACL reconstruction is an option if the child has recurrent instability problems despite completing active rehabilitation or if the child has a secondary intra-articular injury. Therefore, clinicians must work together to closely and frequently monitor the child with repeated MRI and clinical examination as appropriate, being alert to instability episodes and secondary injuries that require prompt assessment and treatment.42
Risks Associated With ACL Reconstruction
Irrespective of the technique, surgical treatment of the ACL has inherent risks. Different ACL reconstruction techniques have different considerations to help avoid risk to the physes, articular surface, and soft tissue structures of the knee. Here we describe 5 key risks associated with surgical treatment for ACL injury of which clinicians, patients, and parents/guardians must be aware.
Risk 1: Growth Disturbance
Growth disturbances are a rare (approximately 2%)41 but serious risk of ACL reconstruction. Growth disturbances may be a result of hardware, bone plugs at the physis, extra-articular tenodesis, or use of over-the-top femoral position. Most of the growth in the child’s lower extremities occurs from the physes of the distal femur and proximal tibia. Any surgical procedures where tunnels are drilled through or near the physis are associated with a risk of growth arrest and associated angular deformity and/or leg-length discrepancy. Transphyseal techniques have a higher rate of graft rupture and a lower rate of lower limb deformity or axis deviation. Physeal-sparing techniques have a lower rate of graft rupture and a higher rate of lower limb deformity or axis deviation.
Highly tensioned soft tissue grafts placed across femoral physes have been associated with limb-length discrepancy and angular deformity.34 Metaphyseal fixation techniques may pose an increased risk of femoral angulation and rotation relative to other techniques. Epiphyseal techniques may increase the risk of rotational deformity and decrease the risk of angular deformity.23 Excessive growth may also be a problem, including symmetrical and asymmetrical overgrowth.22
Most patients with ACL rupture requiring surgical treatment are approaching skeletal maturity and do not have substantial growth remaining. This means that angular deformities and limb-length discrepancies are likely of relatively low clinical significance. Therefore, it may be reasonable to perform transphyseal procedures when the child has minimal growth remaining.
Regularly Monitor the Patient Until Skeletal Maturity
Routine clinical and radiologic follow-up within the first 12 months postoperatively can help the surgeon detect early clinical and radiographic evidence of leg-length discrepancy, angular deformity, or physeal injury. For the child with markedly open physes, appropriate follow-up evaluation of leg-length discrepancy might include annual clinical assessment and knee radiographs with long-leg alignment views until skeletal maturity and physeal closure. Height should be monitored, and if growth exceeds 6 cm in 6 months or if clinical findings warrant, the annual assessment should be brought forward.
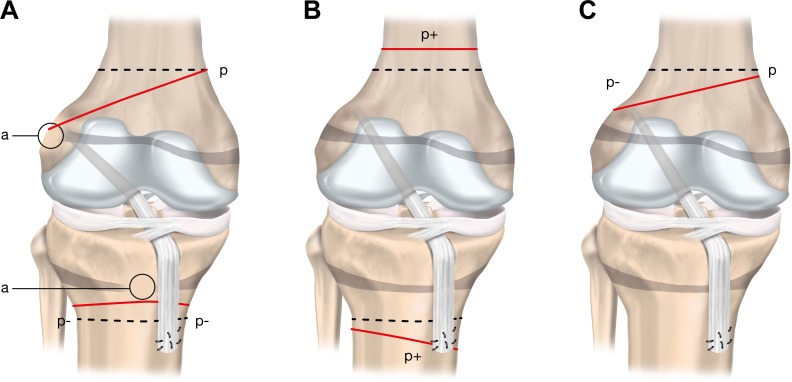
Classifying Growth Disturbances
Growth disturbances can occur in several forms (Figure 9). The growth arrest may be due to
Localized physis injury resulting in a bone bridge leading to growth arrest and possible malalignment (type A)
Overgrowth process potentially caused by hypervascularization (type B)
Undergrowth process arising from a graft traversing a physis under tension during growth and leading to a tethering effect (type C)
Figure 9.
Three growth disturbances that may occur following anterior cruciate ligament (ACL) reconstruction. p represents the physiological growth process; dashed lines represent the physiological growth arrest lines; continuous lines represent the observed pathological growth arrest line. (A) Type A (arrest): growth arrest process (a) occurs due to a localized injury of the physis and results in a bone bridge across the physis. The amount of deformity is proportional to the location and size of the initial physeal injury. (B) Type B (boost): overgrowth process (p+) is probably caused by local hypervascularization, stimulating the open physis. This growth disturbance is temporary and usually becomes apparent in a limited period of 2 years following ACL reconstruction. It primarily leads to leg-length discrepancy. (C) Type C (decelerate): undergrowth process (p–) due to a tenoepiphysiodesis effect. The graft tension across the open physis causes the deformity. Adapted from Chotel et al.22
Risk 2: Secondary ACL Rupture
Young age, returning to pivoting sport, and receiving an allograft are important predictors of new ACL injury after index ACL reconstruction.2,61 One in 4 patients <25 years old who returned to pivoting sports after ACL reconstruction can be expected to sustain a new ACL injury (the pooled ipsilateral reinjury rate is approximately 10%; the pooled contralateral reinjury rate is approximately 12%).135
High rates of reinjury among young people with ACL reconstruction are concerning, although data regarding reinjuries among children with ACL reconstruction are sparse in comparison with data from skeletally mature patients. The best available evidence suggests a graft rupture rate of 13% in children and adolescents (age range, 6-19 years) and a contralateral ACL injury rate of 14%.63 It is reasonable to hypothesize that high-quality rehabilitation with high adherence is likely an important step in reducing reinjury risk. The principles of rehabilitation for the skeletally immature patient are addressed in section 3. The ACL graft is also affected by the status of the other ligaments, menisci, cartilage surfaces, limb alignment, rotation, and the dynamic muscle control of these structures—all factors that must be considered during treatment decision making.
Risk 3: Poor Long-term Knee Health
Meniscectomy is associated with an increased risk for osteoarthritis.24,103,137 Therefore, whenever possible, treatment of ACL injuries must emphasize preservation of the meniscus. Prior meniscectomy at the time of ACL reconstruction is associated with greater likelihood of chondral lesions, while prior meniscal repair is not.19 Because of the technical nature of performing ACL and concurrent meniscal surgery in smaller, younger patients with open physes, patients in whom meniscus repair is indicated should be treated by surgeons who (1) are experienced in treating patients with open physes and (2) perform a high volume of meniscal repairs.
Risk 4: Knee Stiffness
Knee stiffness may be due to the degree of injury to the ACL, disruption of the joint capsule, and injury to structures other than the ACL. Knee stiffness may also be related to surgical interventions or inadequate rehabilitation. Knee stiffness is rare in children aged ≤13 years and is less common in males and in those having surgery with an iliotibial band or hamstring autograft.98 Patients who have knee stiffness following ACL injury should aim for full active knee extension range of motion prior to undergoing ACL reconstruction. If the knee extension deficit persists beyond 3 months postoperatively, MRI may be warranted to assess for anterior impingement (cyclops lesion) and subsequent arthroscopy (should the deficit continue to be unresolved despite focused rehabilitation attention).
Risk 5: Infection
Data related to infection risks for pediatric patients are extrapolated from literature that combines pediatric and adult patients. Infection rates for adult patients are generally low for ACL reconstruction. The rate of deep infections after ACL reconstruction with autograft is 0.19%.13
Management of Associated Injuries
This section addresses the key issues for managing cartilage and meniscal injuries in combination with ACL rupture and the multiligament-injured knee.
Associated Meniscus and Cartilage Injuries in Children With ACL Injuries
The degree of vascular penetration of the menisci declines with age to between 10% and 30% of the menisci receiving vascular inflow in adults.104 The more robust vascular distribution in the pediatric menisci is reflected by increased intrameniscal signal intensity on MRI. Globular and intrameniscal signal may be observed in children and may appear to be an intrasubstance meniscal tear. However, these findings are benign and usually reflect the abundant vascularity of the pediatric menisci (Figure 10).26
Figure 10.
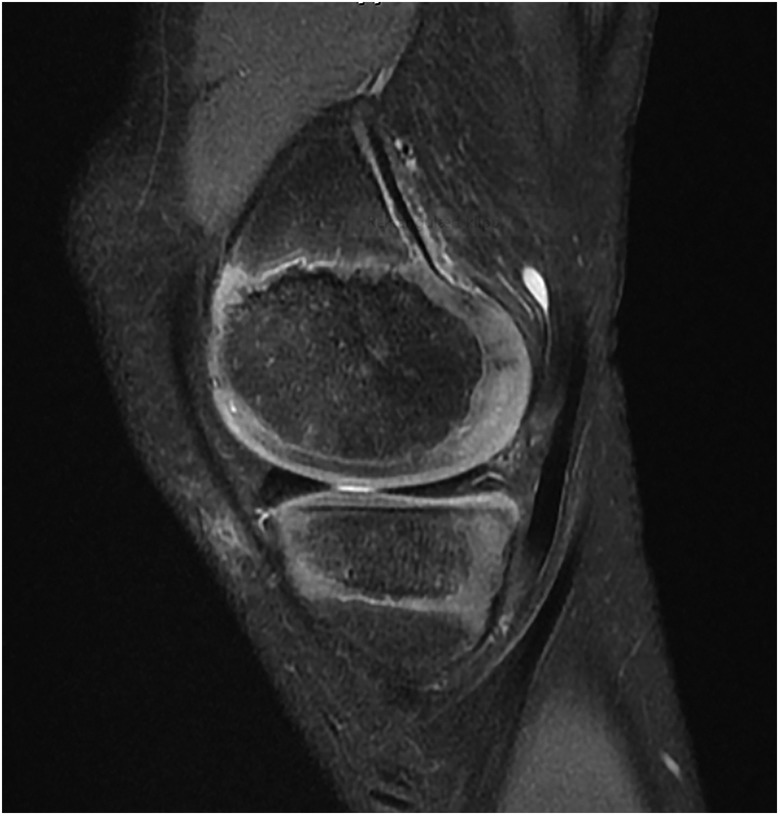
Appearance of the highly vascular pediatric meniscus on magnetic resonance imaging: 10-year-old boy (Signa HDxt 3.0T; GE Medical Systems).
It is important to evaluate the MRI characteristics of the pediatric menisci to rule out meniscal injuries. In cases where the diagnosis is difficult, diagnostic arthroscopy may be performed to clarify the diagnosis and ascertain the state of the meniscus. The clinician should also assess for a posterior medial meniscocapsular tear (ramp lesion). Ramp lesions may be present in 1 in 6 adult patients with ACL injury, and the prevalence of ramp lesions in children with an ACL injury is similar.84 The surgeon should be vigilant to verify the presence or absence of a medial meniscal ramp tear by visualizing the posteromedial compartment. Use a posteromedial knee arthroscopic portal, if necessary, to probe the posteromedial meniscocapsular junction. Ramp lesions may place more stress on an ACL reconstruction if the lesion is not concurrently repaired.30
Meniscal repair should be performed whenever possible in the pediatric patient because of the deleterious effects of meniscectomy and the positive outcomes of meniscal repair (ie, the improved healing potential of the meniscus).4,74,113 This is especially important for bucket-handle, root, and radial meniscal tears and ramp lesions. If the surgeon does not have the skills or equipment to repair the meniscus tear, he or she should consider referring to a surgeon who has the expertise and equipment. Early diagnosis and appropriate treatment of ACL injuries and meniscus tears are needed to provide the best chance of preserving meniscal tissue.
Articular cartilage injuries in combination with ACL injury are less common than meniscal tears.4 However, the clinician should have a higher degree of suspicion of articular cartilage injury in patients with combined ACL and meniscal injuries.33 The medial femoral condyle may be particularly vulnerable.33 Factors that may be associated with more severe chondral lesions are recurrent instability episodes and increased time between ACL injury and reconstruction.33,51,81 It is unclear whether nonsurgical management of ACL injuries is associated with greater incidence of new chondral and meniscal lesions than ACL reconstruction.92
Associated Ligament Injuries in Children With ACL Injuries
There is limited research on multiligament knee injuries and treatment in pediatric patients, and these injuries are less common in children than in adults.87 Therefore, consider referral to a specialist center.
Specific Surgical Treatment Considerations
Combined ACL and Fibular Collateral Ligament Injuries
Use fluoroscopy prior to placing suture anchors for a repair or for tunnel reaming for a concurrent ligament reconstruction to evaluate tunnel position in relation to the physes.136
Combined ACL and Posterior Cruciate Ligament Injuries
Nonsurgical treatment may be appropriate for partial posterior cruciate ligament (PCL) tears or nondisplaced avulsion injuries. PCL reconstruction is a relatively safe and viable treatment option for patients with multiligament injuries.69 Using a tibial inlay technique with a modified femoral tunnel location avoids transphyseal drilling.132 However, there are no high-quality studies of this technique in children.
True Knee Dislocation
Perform a reduction by manipulating the tibia relative to the femur. Avoid forceful hypertension or rotation to minimize the risk for damage to cartilaginous and/or neurovascular structures. Following reduction, a dynamic knee brace can be applied (for at least 12 weeks) to prevent further intra-articular damage and to help hold the knee in a reduced position79 while further treatment is planned. Ultimately, reconstruction of the ACL and PCL in combination with repair/reconstruction of additional ligaments (as needed) is the appropriate treatment.
Section 5: Pediatric Patient-Reported Outcome Measures
This section addresses the fundamental clinical question, How does the clinician measure outcomes that are relevant to the child with an ACL injury? Assessing patient-reported outcome measures (PROMs) provides insight into aspects of the patient’s function that cannot be evaluated with clinical tests or imaging.27 Because of this, evaluating PROMs is important when managing the child with an ACL injury and when conducting research in this field.
Valid outcome instruments must have appropriate measurement properties, including reliability, validity (content, criterion, and construct), and responsiveness. Instruments that were developed for adults may not be valid for children and adolescents. Pediatric patients have different levels of comprehension (this age group includes a spectrum of comprehension abilities from younger children to older adolescents) and interpretation of instruments. Most important, pediatric patients may value different outcomes when evaluating their knee function, and instruments must reflect the issues that are important to children and adolescents.
Pediatric PROMs should be either developed or specifically validated in this population. The process of validation should include an assessment of comprehensibility, reliability, validity, and responsiveness. Child-reported outcome assessment is typically valid for older children and adolescents (≥10 years).116 For younger children (<10 years), parent proxy–reported outcome assessment may be more appropriate. However, there is potential for bias with proxy-reported outcomes.109
Pediatric PROMs (Table 6) must be valid for children and adolescents with ACL injury. However, a pediatric-derived PROM is not currently available. Such an instrument would ensure that the items covered issues that matter most to children and adolescents. The Pediatric International Knee Documentation Committee (Pedi-IKDC) and Knee-injury and Osteoarthritis Outcome Score for Children (KOOS-Child) were adapted from adult PROMs designed to assess self-reported knee function. The Pedi-IKDC has been correlated with the IKDC subjective knee form, providing preliminary evidence of construct validity.10,11 Given that patients with a history of ACL injury may develop symptoms and signs of osteoarthritis within 10 years of the index injury60 and given the relationship between symptomatic osteoarthritis and poor quality of life,134 assessing quality of life and long-term knee function outcomes with valid PROMs may also be important.
TABLE 6.
Appropriate PROMs for the Child With ACL Injurya
| Type of Instrument | Scale |
|---|---|
| Health-related quality of life | Child Health Questionnaire56 PedsQL129 Pediatric PROMIS57 |
| Condition or region specific | Pedi-IKDC70 KOOS-Child99 |
| Activity-level assessment | Pediatric Functional Activity Brief Scale39 |
aACL, anterior cruciate ligament; IKDC, International Knee Documentation Committee; KOOS, Knee injury and Osteoarthritis Outcome Score; PedsQL, Pediatric Quality of Life inventory; PROMs, patient-reported outcome measures; PROMIS, patient-reported outcomes measurement information system.
Recommendations for using PROMs in clinical practice with pediatric patients include the following:
A generic measure of health-related quality of life
Either the Pedi-IKDC or KOOS-Child to assess self-reported knee function
The Pediatric Functional Activity Brief Scale to assess self-reported activity level
In research, it may be appropriate to include other PROMs depending on the research question. Researchers need to make decisions about the most appropriate outcomes when planning their study.
Section 6: Ethical Considerations
This section addresses the fundamental clinical question, What are the clinician’s roles and responsibilities? Treatment decisions that involve children are among the most difficult decisions that the clinician faces, especially when scientific knowledge is limited. Striking a balance among ethical principles can be especially challenging when there is a conflict of opinion. In this section, we outline the relevant ethical considerations for the clinician who treats children with ACL injuries.
It is impossible to provide specific ethical guidance that applies to all sporting injuries in adolescents and children, given the varying individual circumstances. However, it is incontrovertible that it is in the best interests of all children not to have knee and associated injuries. Therefore, injury prevention programs are fundamental to the best interests of the child. Clinicians have an obligation to support policies and practices that encourage coaches, teams/clubs, and national and international federations to prioritize injury prevention. All parties should be committed to protecting the long-term welfare of the growing child. Nevertheless, there may be exceptional cases where parents/guardians may, with the approval of their child, rationally prioritize short-term goals. One example could be that, despite inherent risks for reinjury, an early return to sport might be a high priority for a child who has exceptional talent in a given sport.
Protecting the integrity of the knee should be the clinician’s primary focus. Decisions regarding how to protect the integrity of the child’s knee must be shared among the child, parent/guardian (surrogate decision maker), and clinician.18 Parents have an obligation to care for their children and bring them up to live good lives.17 Nevertheless, parents have different perceptions of what constitutes “good living.”20 Most ethicists agree that parental influence is a positive thing.14 However, in high-performance children’s sport, parents and coaches can pressure the child and clinician to focus on short-term athletic goals at the expense of long-term welfare.54
Issues Related to Consent and Obtaining Consent for Treatment
Children are a vulnerable population.6,44 In the context of treatment of ACL injury, the child is doubly vulnerable given his or her developing but uncertain life plans78 and developmental stage. We can never be certain of all the risks to the normal development of the individual child.85 It is difficult to gain legally legitimate informed consent from children in the treatment decision-making process. Therefore, the clinician needs to act as a cofiduciary on behalf of the child, while parents give consent.88
The clinician and/or parents are obliged to serve the interests of the child above all other interests.88,121 This is what is meant by having a fiduciary duty to the patient. The clinician must talk with the child and the surrogate decision makers in ways that are respectful of and comprehensible by everyone involved.1 In addition to avoiding conflicts of interest, the clinician must always seek the approval or assent of the child, irrespective of the wishes of the parent/guardian, at a communication level that matches the child’s competence.43 The child should be present in all discussions concerning him or her, to respect his or her (emerging) autonomy.131
Arriving at a Shared Decision
There should be consensus among all parties when arriving at a decision. This consensus should be based on realistic assessments of risks and benefits and a proper consideration of the goals of the child and parent. The clinician’s responsibility is to guide this discussion with accurate information from the best-quality research. Several ethical standards can help the clinician, child, and parents navigate the decision-making process and arrive at ethically justified treatment decisions.
Pediatric ethical standards are not identical: some aim at higher thresholds, while others accept a lower threshold of justification. Six standards can be helpful in different clinical scenarios in pediatric ACL injury:
Best interests 72: Widely used, but it is difficult to predict what is in the best long-term interests of a child
Harm principle 31: A threshold below which the clinician should not acquiesce to parent-led decision so that the child is not harmed
Parental discretion 45,64: Parent preference is accepted because it is not sufficiently harmful to the child for the clinician to dissent from the parent’s choice
Costs/benefits 29: Involves risk assessment, but its application to the child means that the clinician may need to compare very different kinds of futures that may or may not eventuate
Not unreasonable 105: Focuses only on the appropriateness of decisions and decision makers
Reasonable choice 96: A decision method that attempts to incorporate the previous 5 standards into a single model or intervention
The clinician has an important role in treatment decision making because he or she typically has superior knowledge of treatment options, risk, and benefits than do children and parents. To best guide the child and his or her parents, the clinician must have a clear idea of the range of interventions that are optimal, acceptable, and not desirable, and he or she must be able to justify this with reference to the best-quality research and clinical experience. In many health care settings, parents take responsibility for the ACL treatment decision, commensurate with the child’s assent. Where there is a lack of consensus in the decision-making process (eg, the parent decides in favor of something that is not recommended by the clinician), the clinician may also consider whether he or she can defend a treatment recommendation based on 1 of the 6 ethical standards.
Section 7: Future Research
Management of pediatric ACL injuries is highly debated. Reflecting some of the concern and controversy is a high ratio of clinical commentaries and narrative reviews to original articles on this topic. The problem for the clinician is that high-quality evidence is scarce to help him or her best manage pediatric ACL injuries. The scientific literature is inconsistent and limited by inferior methods that carry a high risk of bias.35,91 There are no randomized trials comparing different treatment approaches or surgical techniques. Most publications have only short-term follow-up—none beyond 10 years. Therefore, long-term knee health (including osteoarthritis) and quality of life are unknown.
Methodological Considerations
Future studies must address 5 key issues:
Most clinical studies on pediatric ACL injury are of cross-sectional or retrospective design, and the study populations are often at high risk of selection bias and include small samples. This means that there is a high risk that existing research does not reflect the typical pediatric patient with an ACL injury.
Many studies do not provide adequate descriptions of the treatments that the patients have received, and patient adherence has not been reported. A meaningful interpretation of study outcomes is possible only with a detailed description of the surgical technique, rehabilitation, brace usage, return-to-sport clearance, and recommendations of activity modification.
Many studies fail to assess the skeletal age of included participants, and few report the remaining growth of participants. Chronological age alone is an unreliable indicator of skeletal maturity. Because of this, it is difficult to know to which skeletal age group these research results apply.
Patients aged up to 18 years are often included in pediatric studies. This is a problem because it is likely that the patient population is a mix of skeletally mature and immature patients. Therefore, the literature may be biased toward the older patients. Having mixed populations also complicates pooling or comparing results from skeletally immature patients across studies.
Knowledge of preinjury and posttreatment activity level gives important insight into a key risk factor for injury. The greater exposure that a child has to potentially injurious situations (eg, playing pivoting sports), the greater the chance of injury (or reinjury). Activity level is a key confounding factor that is rarely accounted for in statistical analyses. This means that there is a risk that estimates of secondary injury incidence may be over- or underestimated in comparisons among studies or patient groups.
Research Priorities
There are 4 research priority areas to improve prevention and outcomes of pediatric ACL injury:
Prospective injury surveillance studies to identify injury mechanisms and modifiable risk factors for ACL injury, combined injuries, and knee reinjuries.
Prospective research on outcomes after surgical and nonsurgical treatment (active rehabilitation alone). Long-term follow-up (beyond 10 years) is essential to answer key questions of how an ACL injury in childhood affects physical activity, future knee health, and quality of life.
Research on the efficacy of different surgical techniques and characteristics (eg, timing of surgery, graft types) and active rehabilitation programs, knee brace use, and activity modification after injury and surgery.
Multicenter and registry studies should be prioritized. Because of smaller numbers of ACL injuries in pediatric patients than in skeletally mature patients, specialist treatment centers, expert clinicians, and researchers must prioritize collaboration.
Acknowledgment
Our sincere thanks to Cherine Touvet-Fahmy and Fiona Trabelsi from the IOC Medical and Scientific Department for their help and support with all arrangements ahead of and during the Lausanne consensus meeting. Our thanks to Pontus Andersson from Pontus Art Production, Gothenburg, Sweden, for the illustrations. We gratefully acknowledge the contribution and support of the IOC Medical and Scientific Chair, Dr Uğur Erdener, during the consensus meeting and the IOC for funding the meeting. Håvard Moksnes acknowledges Olympiatoppen Norway and Idrettens Helsesenter, Oslo, Norway.
Appendix
TABLE A1.
Delphi Consensus Process Statements
|
TABLE A2.
Exercise Examples for Each Phase of Pediatric Anterior Cruciate Ligament Rehabilitation
| Phase 1 |
|
| Phase 2 |
|
| Phase 3 |
|
| Phase 4 | Injury prevention (refer to Section 1 of the consensus statement, and FIFA 11+ for Kids manual1 for guidance) |
In Memory of Dr Allen Anderson: An excellent clinician-scientist and a keen coworker in this project, Allen F. Anderson, MD, died in a farming accident on Sunday, November 12, 2017. This tragedy occurred shortly after he had been an active participant in this IOC consensus meeting on the topic of his lifelong clinical and research passion: pediatric ACL injuries. Born on November 16, 1949, Dr Anderson was a graduate of the University of Tennessee College of Medicine. He completed a residency in orthopaedics at Vanderbilt University and was board certified by the American Board of Orthopaedic Surgery in general orthopaedics, with a certificate of added qualification for sports medicine. Dr Anderson was a sports medicine specialist with an interest in knee injury and ligament reconstruction and with special interest in children’s injuries. He published >100 peer-reviewed journal articles and 26 book chapters and received a patent for the invention of a pediatric ACL reconstruction system. Among numerous awards are 3 standouts: being recognized as one of America’s Top Physicians (2004-2012) from the Consumers’ Research Council, being elected to Best Doctors in America by his peers (2007-2008), and being Nashville Business Journal Top Doctor (2016-2017).
Dr Anderson had many prestigious positions through his life. He served as president of the AOSSM from 2015 to 2016 and as an associate editor of The Orthopaedic Journal of Sports Medicine and The American Journal of Sports Medicine. Above all, he was a true friend and colleague to whom you could go with problems and challenges, not the least among our youngest patients. Allen will be greatly missed by us all.
This article has been co-published in the British Journal of Sports Medicine, Journal of ISAKOS, and Knee Surgery, Sports Traumatology, Arthroscopy. Minor differences exist between this version and the others to be consistent with OJSM editorial style.
One or more of the authors has declared the following potential conflicts of interest or source of funding: M.C. is a paid consultant for Arthrex. M.S.K. is a paid consultant for Best Doctors, OrthoPediatrics, Össur, and Smith & Nephew; receives royalties, financial, or material support from OrthoPediatrics, Össur, Saunders/Mosby-Elsevier, and Wolters Kluwer Health–Lippincott Williams & Wilkins; and is a paid member of the Steadman Philippon Research Institute Scientific Advisory Committee. R.F.L. receives royalties from Össur, Arthrex, and Smith & Nephew. B.R. receives royalties from Elsevier, salary from The American Journal of Sports Medicine and The Orthopaedic Journal of Sports Medicine, holds stock/stock options in Merck and Johnson & Johnson, and is editor in chief of The Orthopaedic Journal of Sports Medicine. R. Seil is an unpaid board member and president of the European Society of Sports Traumatology Knee Surgery and Arthroscopy (ESSKA). T.S. is the scientific manager in the Medical and Scientific Department of the International Olympic Committee. L.E. is the head of scientific activities in the Medical and Scientific Department of the International Olympic Committee, has received speaking fees from Smith & Nephew, receives research funding from Biomet and Smith & Nephew, has received employment funds from Arthrex and Smith & Nephew, and receives royalties or consulting fees from Arthrex.
References
- 1. Alderson P. Children’s Consent to Surgery. Buckingham, England: Open University Press; 1993. [Google Scholar]
- 2. Andernord D, Desai N, Björnsson H, et al. Predictors of contralateral anterior cruciate ligament reconstruction: a cohort study of 9061 patients with 5-year follow-up. Am J Sports Med. 2015;43:295–302. [DOI] [PubMed] [Google Scholar]
- 3. Anderson AF. Transepiphyseal replacement of the anterior cruciate ligament using quadruple hamstring grafts in skeletally immature patients. J Bone Joint Surg Am. 2004;86(suppl 1, pt 2):201–209. [DOI] [PubMed] [Google Scholar]
- 4. Anderson AF, Anderson CN. Correlation of meniscal and articular cartilage injuries in children and adolescents with timing of anterior cruciate ligament reconstruction. Am J Sports Med. 2015;43:275–281. [DOI] [PubMed] [Google Scholar]
- 5. Andrews M, Noyes FR, Barber-Westin SD. Anterior cruciate ligament allograft reconstruction in the skeletally immature athlete. Am J Sports Med. 1994;22:48–54. [DOI] [PubMed] [Google Scholar]
- 6. Archard D. Children: Rights and Childhood. Abingdon, England: Routledge; 2014. [Google Scholar]
- 7. Ardern CL, Glasgow P, Schneiders A, et al. 2016 consensus statement on return to sport from the First World Congress in Sports Physical Therapy, Bern. Br J Sports Med. 2016;50:853–864. [DOI] [PubMed] [Google Scholar]
- 8. Ardern CL, Österberg A, Tagesson S, et al. The impact of psychological readiness to return to sport and recreational activities after anterior cruciate ligament reconstruction. Br J Sports Med. 2014;48:1613–1619. [DOI] [PubMed] [Google Scholar]
- 9. Ardern CL, Webster KE, Taylor NF, et al. Return to sport following anterior cruciate ligament reconstruction surgery: a systematic review and meta-analysis of the state of play. Br J Sports Med. 2011;45:596–606. [DOI] [PubMed] [Google Scholar]
- 10. Astur DC, Arliani GG, Debieux P, et al. Intraarticular hamstring graft diameter decreases with continuing knee growth after ACL reconstruction with open physes. Knee Surg Sports Traumatol Arthrosc. 2016;24:792–795. [DOI] [PubMed] [Google Scholar]
- 11. Astur DC, Cachoeira CM, da Silva Vieira T, et al. Increased incidence of anterior cruciate ligament revision surgery in paediatric versus adult population [published online September 25, 2017]. Knee Surg Sports Traumatol Arthrosc. doi:10.1007/s00167-017-4727-z [DOI] [PubMed] [Google Scholar]
- 12. Attwood MJ, Roberts SP, Trewartha G, et al. Efficacy of a movement control injury prevention programme in adult men’s community rugby union: a cluster randomised controlled trial. Br J Sports Med. 2018;52(6):368–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bansal A, Lamplot JD, VandenBerg J, et al. Meta-analysis of the risk of infections after anterior cruciate ligament reconstruction by graft type [published online July 1, 2017]. Am J Sports Med. doi:10.1177/0363546517714450 [DOI] [PubMed] [Google Scholar]
- 14. Beauchamp TL, Childress JF. Principles of Biomedical Ethics. New York, NY: Oxford University Press; 2001. [Google Scholar]
- 15. Bergeron MF, Mountjoy M, Armstrong N, et al. International Olympic Committee consensus statement on youth athletic development. Br J Sports Med. 2015;49:843–851. [DOI] [PubMed] [Google Scholar]
- 16. Bollen S, Pease F, Ehrenraich A, et al. Changes in the four-strand hamstring graft in anterior cruciate ligament reconstruction in the skeletally-immature knee. J Bone Joint Surg Br. 2008;90:455–459. [DOI] [PubMed] [Google Scholar]
- 17. Brighouse H, Swift A. Family Values: The Ethics of Parent-Child Relationships. Princeton, NJ: Princeton University Press; 2014. [Google Scholar]
- 18. Brock DW. The ideal of shared decision making between physicians and patients. Kennedy Institute of Ethics Journal. 1991;1:28–47. [DOI] [PubMed] [Google Scholar]
- 19. Brophy RH, Wright RW, David TS, et al. Association between previous meniscal surgery and the incidence of chondral lesions at revision anterior cruciate ligament reconstruction. Am J Sports Med. 2012;40:808–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Buchanan AE, Brock DW. Deciding for Others: The Ethics of Surrogate Decisionmaking. Cambridge, UK: Cambridge University Press; 1989. [Google Scholar]
- 21. Calvo R, Figueroa D, Gili F, et al. Transphyseal anterior cruciate ligament reconstruction in patients with open physes: 10-year follow-up study. Am J Sports Med. 2015;43:289–294. [DOI] [PubMed] [Google Scholar]
- 22. Chotel F, Henry J, Seil R, et al. Growth disturbances without growth arrest after ACL reconstruction in children. Knee Surg Sports Traumatol Arthrosc. 2010;18:1496–1500. [DOI] [PubMed] [Google Scholar]
- 23. Chudik S, Beasley L, Potter H, et al. The influence of femoral technique for graft placement on anterior cruciate ligament reconstruction using a skeletally immature canine model with a rapidly growing physis. Arthroscopy. 2007;23:1309–1319. [DOI] [PubMed] [Google Scholar]
- 24. Cohen M, Amaro JT, Ejnisman B, et al. Anterior cruciate ligament reconstruction after 10 to 15 years: association between meniscectomy and osteoarthrosis. Arthroscopy. 2007;23:629–634. [DOI] [PubMed] [Google Scholar]
- 25. Cohen M, Ferretti M, Quarteiro M, et al. Transphyseal anterior cruciate ligament reconstruction in patients with open physes. Arthroscopy. 2009;25:831–838. [DOI] [PubMed] [Google Scholar]
- 26. Crues JV, 3rd, Mink J, Levy TL, et al. Meniscal tears of the knee: accuracy of MR imaging. Radiology. 1987;164:445–448. [DOI] [PubMed] [Google Scholar]
- 27. Davis JC, Bryan S. Patient reported outcome measures (PROMs) have arrived in sports and exercise medicine: why do they matter? Br J Sports Med. 2015;49:1545–1546. [DOI] [PubMed] [Google Scholar]
- 28. Dekker TJ, Godin JA, Dale KM, et al. Return to sport after pediatric anterior cruciate ligament reconstruction and its effect on subsequent anterior cruciate ligament injury. J Bone Joint Surg Am. 2017;99:897–904. [DOI] [PubMed] [Google Scholar]
- 29. DeMarco JP, Powell DP, Stewart DO. Best interest of the child: surrogate decision making and the economics of externalities. J Bioeth Inq. 2011;8:289–298. [Google Scholar]
- 30. DePhillipo NN, Cinque ME, Chahla J, et al. Incidence and detection of meniscal ramp lesions on magnetic resonance imaging in patients with anterior cruciate ligament reconstruction. Am J Sports Med. 2017;45:2233–2237. [DOI] [PubMed] [Google Scholar]
- 31. Diekema DS. Parental refusals of medical treatment: the harm principle as threshold for state intervention. Theor Med Bioeth. 2004;25:243–264. [DOI] [PubMed] [Google Scholar]
- 32. Donaldson A, Cook J, Gabbe B, et al. Bridging the gap between content and context: establishing expert consensus on the content of an exercise training program to prevent lower-limb injuries. Clin J Sport Med. 2015;25:221–229. [DOI] [PubMed] [Google Scholar]
- 33. Dumont GD, Hogue GD, Padalecki JR, et al. Meniscal and chondral injuries associated with pediatric anterior cruciate ligament tears: relationship of treatment time and patient-specific factors. Am J Sports Med. 2012;40:2128–2133. [DOI] [PubMed] [Google Scholar]
- 34. Edwards TB, Greene CC, Baratta RV, et al. The effect of placing a tensioned graft across open growth plates: a gross and histologic analysis. J Bone Joint Surg Am. 2001;83:7725–7734. [DOI] [PubMed] [Google Scholar]
- 35. Ekås GR, Ardern CL, Grindem H, et al. New meniscal tears after ACL injury: what is the risk? A systematic review protocol. Br J Sports Med. 2018;52(6):386. [DOI] [PubMed] [Google Scholar]
- 36. Emery CA, Roy T-O, Whittaker JL, et al. Neuromuscular training injury prevention strategies in youth sport: a systematic review and meta-analysis. Br J Sports Med. 2015;49:865–870. [DOI] [PubMed] [Google Scholar]
- 37. Eubank BH, Mohtadi NG, Lafave MR, et al. Using the modified Delphi method to establish clinical consensus for the diagnosis and treatment of patients with rotator cuff pathology. BMC Med Res Methodol. 2016;16:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Fabricant PD, Kocher MS. Management of ACL injuries in children and adolescents. J Bone Joint Surg Am. 2017;99:600–612. [DOI] [PubMed] [Google Scholar]
- 39. Fabricant PD, Robles A, Downey-Zayas T, et al. Development and validation of a pediatric sports activity rating scale: the Hospital for Special Surgery Pediatric Functional Activity Brief Scale (HSS Pedi-FABS). Am J Sports Med. 2013;41:2421–2429. [DOI] [PubMed] [Google Scholar]
- 40. Fleming BC, Spindler KP, Palmer MP, et al. Collagen-platelet composites improve the biomechanical properties of healing anterior cruciate ligament grafts in a porcine model. Am J Sports Med. 2009;37:1554–1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Frosch KH, Stengel D, Brodhun T, et al. Outcomes and risks of operative treatment of rupture of the anterior cruciate ligament in children and adolescents. Arthroscopy. 2010;26:1539–1550. [DOI] [PubMed] [Google Scholar]
- 42. Funahashi KM, Moksnes H, Maletis GB, et al. Anterior cruciate ligament injuries in adolescents with open physis: effect of recurrent injury and surgical delay on meniscal and cartilage injuries. Am J Sports Med. 2014;42:1068–1073. [DOI] [PubMed] [Google Scholar]
- 43. Gert B, Clouser KD, Culver C. Bioethics: A Return to Fundamentals. New York, NY: Oxford University Press; 1997. [Google Scholar]
- 44. Gheaus A. Children’s vulnerability and legitimate authority over children. J Appl Philos. 2018;35:60–75. [Google Scholar]
- 45. Gillam L. The zone of parental discretion: an ethical tool for dealing with disagreement between parents and doctors about medical treatment for a child. Clin Ethics. 2016;11:1–8. [Google Scholar]
- 46. Gilsanz V, Ratib O. Hand Bone Age: A Digital Atlas of Skeletal Maturity. Heidelberg, Germany: Springer-Verlag; 2011. [Google Scholar]
- 47. Goddard M, Bowman N, Salmon LJ, et al. Endoscopic anterior cruciate ligament reconstruction in children using living donor hamstring tendon allografts. Am J Sports Med. 2013;41:567–574. [DOI] [PubMed] [Google Scholar]
- 48. Greulich W, Pyle SI. Radiographic Atlas of Skeletal Development of the Hand and Wrist. Stanford, CA: Stanford University Press; 1959. [Google Scholar]
- 49. Grindem H, Eitzen I, Engebretsen L, et al. Nonsurgical or surgical treatment of ACL injuries: knee function, sports participation, and knee reinjury: the Delaware-Oslo ACL Cohort Study. J Bone Joint Surg Am. 2014;96:1233–1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Grindem H, Snyder-Mackler L, Moksnes H, et al. Simple decision rules can reduce reinjury risk by 84% after ACL reconstruction: the Delaware-Oslo ACL Cohort Study. Br J Sports Med. 2016;50:804–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Guenther ZD, Swami V, Dhillon SS, et al. Meniscal injury after adolescent anterior cruciate ligament injury: how long are patients at risk? Clin Orthop Relat Res. 2014;472:990–997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Hägglund M, Atroshi I, Wagner P, et al. Superior compliance with a neuromuscular training programme is associated with fewer ACL injuries and fewer acute knee injuries in female adolescent football players: secondary analysis of an RCT. Br J Sports Med. 2013;47:974–979. [DOI] [PubMed] [Google Scholar]
- 53. Henry J, Chotel F, Chouteau J, et al. Rupture of the anterior cruciate ligament in children: early reconstruction with open physes or delayed reconstruction to skeletal maturity? Knee Surg Sports Traumatol Arthrosc. 2009;17:748–755. [DOI] [PubMed] [Google Scholar]
- 54. Holt NL, Knight CJ. Parenting in Youth Sport: From Research to Practice. Abingdon, England: Routledge; 2014. [Google Scholar]
- 55. Hui C, Roe J, Ferguson D, et al. Outcome of anatomic transphyseal anterior cruciate ligament reconstruction in Tanner stage 1 and 2 patients with open physes. Am J Sports Med. 2012;40:1093–1098. [DOI] [PubMed] [Google Scholar]
- 56. Hullmann SE, Ryan JL, Ramsey RR, et al. Measures of general pediatric quality of life: Child Health Questionnaire (CHQ), DISABKIDS Chronic Generic Measure (DCGM), KINDL-R, Pediatric Quality of Life Inventory (PedsQL) 4.0 Generic Core Scales, and Quality of My Life Questionnaire (QoML). Arthritis Care Res (Hoboken). 2011;63(suppl 11):S420–S430. [DOI] [PubMed] [Google Scholar]
- 57. Irwin DE, Varni JW, Yeatts K, et al. Cognitive interviewing methodology in the development of a pediatric item bank: a Patient Reported Outcomes Measurement Information System (PROMIS) study. Health Qual Life Outcomes. 2009;23:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Janarv PM, Wikstrom B, Hirsch G. The influence of transphyseal drilling and tendon grafting on bone growth: an experiment study in the rabbit. J Pediatric Orthop. 1998;18:149–154. [PubMed] [Google Scholar]
- 59. Johnsen MB, Eitzen I, Moksnes H, et al. Inter- and intrarater reliability of four single-legged hop tests and isokinetic muscle torque measurements in children. Knee Surg Sports Traumatol Arthrosc. 2015;23:1907–1916. [DOI] [PubMed] [Google Scholar]
- 60. Johnson VL, Roe JP, Salmon LJ, et al. Does age influence the risk of incident knee osteoarthritis after a traumatic anterior cruciate ligament injury? Am J Sports Med. 2016;44:2399–2405. [DOI] [PubMed] [Google Scholar]
- 61. Kaeding CC, Pedroza ED, Reinke EK, et al. Risk factors and predictors of subsequent ACL injury in either knee after ACL reconstruction: prospective analysis of 2488 primary ACL reconstructions from the MOON cohort. Am J Sports Med. 2015;43:1583–1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Kaplan PA, Nelson NL, Garvin KL, et al. MR of the knee: the significance of high signal in the meniscus that does not clearly extend to the surface. AJR Am J Roentgenol. 1991;156:333–336. [DOI] [PubMed] [Google Scholar]
- 63. Kay J, Memon M, Marx RG, Peterson D, Simunovic N, Ayeni OR. Over 90% of children and adolescents return to sport after anterior cruciate ligament reconstruction: a systematic review and meta-analysis [published online January 13, 2018]. Knee Surg Sports Traumatol Arthrosc. doi:10.1007/s00167-018-4830-9 [DOI] [PubMed] [Google Scholar]
- 64. Kilham H, Isaacs D, Kerridge I. When refusal is only distantly or unpredictably life-threatening In: McDougall R, Delaney C, Gillam L, eds. When Doctors and Parents Disagree: Ethics, Paediatrics and the Zone of Parental Discretion. Sydney, Australia: Federation Press; 2016. [Google Scholar]
- 65. Kocher MS, DiCanzio J, Zurakowski D, et al. Diagnostic performance of clinical examination and selective magnetic resonance imaging in the evaluation of intraarticular knee disorders in children and adolescents. Am J Sports Med. 2001;29:292–296. [DOI] [PubMed] [Google Scholar]
- 66. Kocher MS, Garg S, Micheli LJ. Physeal sparing reconstruction of the anterior cruciate ligament in skeletally immature prepubescent children and adolescents. J Bone Joint Surg Am. 2005;87:2371–2379. [DOI] [PubMed] [Google Scholar]
- 67. Kocher MS, Garg S, Micheli LJ. Physeal sparing reconstruction of the anterior cruciate ligament in skeletally immature prepubescent children and adolescents: surgical technique. J Bone Joint Surg Am. 2006;88(suppl 1, pt 2):283–293. [DOI] [PubMed] [Google Scholar]
- 68. Kocher MS, Saxon HS, Hovis WD, et al. Management and complications of anterior cruciate ligament injuries in skeletally immature patients: survey of the Herodicus Society and the ACL Study Group. J Pediatric Orthop. 2002;22:452–457. [PubMed] [Google Scholar]
- 69. Kocher MS, Shore B, Nasreddine AY, et al. Treatment of posterior cruciate ligament injuries in pediatric and adolescent patients. J Pediatric Orthop. 2012;32:553–560. [DOI] [PubMed] [Google Scholar]
- 70. Kocher MS, Smith JT, Iversen MD, et al. Reliability, validity, and responsiveness of a modified International Knee Documentation Committee Subjective Knee Form (Pedi-IKDC) in children with knee disorders. Am J Sports Med. 2001;39:933–939. [DOI] [PubMed] [Google Scholar]
- 71. Kocher MS, Smith JT, Zoric BJ, et al. Transphyseal anterior cruciate ligament reconstruction in skeletally immature pubescent adolescents. J Bone Joint Surg Am. 2007;89:2632–2639. [DOI] [PubMed] [Google Scholar]
- 72. Kopelman LM. The best-interests standard as threshold, ideal, and standard of reasonableness. J Med Phiols. 1997;22:271–289. [DOI] [PubMed] [Google Scholar]
- 73. Kopf S, Schenkengel JP, Wieners G, et al. No bone tunnel enlargement in patients with open growth plates after transphyseal ACL reconstruction. Knee Surg Sports Traumatol Arthrosc. 2010;18:1445–1451. [DOI] [PubMed] [Google Scholar]
- 74. Krych AJ, McIntosh AL, Voll AE, et al. Arthroscopic repair of isolated meniscal tears in patients 18 years and younger. Am J Sports Med. 2008;39:1283–1289. [DOI] [PubMed] [Google Scholar]
- 75. Krych AJ, Pitts RT, Dajani KA, et al. Surgical repair of meniscal tears with concomitant anterior cruciate ligament reconstruction in patients 18 years and younger. Am J Sports Med. 2010;38:976–982. [DOI] [PubMed] [Google Scholar]
- 76. Kvist J, Kartus J, Karlsson J, Forssblad M. Results from the Swedish national anterior cruciate ligament register. Arthroscopy. 2014;30:803–810. [DOI] [PubMed] [Google Scholar]
- 77. Kyritsis P, Bahr R, Landreau P, et al. Likelihood of ACL graft rupture: not meeting six clinical discharge criteria before return to sport is associated with a four times greater risk of rupture. Br J Sports Med. 2016;50:946–951. [DOI] [PubMed] [Google Scholar]
- 78. Langford G. Education, Persons and Society. London, England: Macmillan; 1985. [Google Scholar]
- 79. LaPrade RF, Smith SD, Wilson KJ, et al. Quantification of functional brace forces for posterior cruciate ligament injuries on the knee joint: an in vivo investigation. Knee Surg Sports Traumatol Arthrosc. 2015;23:3070–3076. [DOI] [PubMed] [Google Scholar]
- 80. Lauersen JB, Bertelsen DM, Andersen LB. The effectiveness of exercise interventions to prevent sports injuries: a systematic review and meta-analysis of randomised controlled trials. Br J Sports Med. 2014;48:871–877. [DOI] [PubMed] [Google Scholar]
- 81. Lawrence JT, Argawal N, Ganley TJ. Degeneration of the knee joint in skeletally immature patients with a diagnosis of an anterior cruciate ligament tear: is there harm in delay of treatment? Am J Sports Med. 2011;39:2582–2587. [DOI] [PubMed] [Google Scholar]
- 82. Lo IK, Kirkley A, Fowler PJ, et al. The outcome of operatively treated anterior cruciate ligament disruptions in the skeletally immature child. Arthroscopy. 1997;13:627–634. [DOI] [PubMed] [Google Scholar]
- 83. Magarian EM, Vavken P, Murray MM. Human anterior cruciate ligament fibroblasts from immature patients have a stronger in vitro response to platelet concentrates than those from mature individuals. Knee. 2011;18:247–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Malatray M, Raux S, Peltier A, Pfirrmann C, Seil R, Chotel F. Ramp lesions in ACL deficient knees in children and adolescent population: a high prevalence confirmed in intercondylar and posteromedial exploration [published online March 22, 2017]. Knee Surg Sports Traumatol Arthrosc. doi:10.1007/s00167-017-4471-4 [DOI] [PubMed] [Google Scholar]
- 85. Malina RM. Growth In: Mooren FC, ed. Encyclopedia of Exercise Medicine in Health and Disease. Heidelberg, Germany: Springer; 2012:376–378. [Google Scholar]
- 86. Mandelbaum BR, Silvers HJ, Watanabe D, et al. Effectiveness of a neuromuscular and proprioceptive training program in preventing anterior cruciate ligament injuries in female athletes: 2-year follow-up. Am J Sports Med. 2005;33:1003–1010. [DOI] [PubMed] [Google Scholar]
- 87. Mayer S, Albright JC, Stoneback JW. Pediatric knee dislocations and physeal fractures about the knee. J Am Acad Orthop Surg. 2015;23:571–580. [DOI] [PubMed] [Google Scholar]
- 88. McCullough LB. Contributions of ethical theory to pediatric ethics: pediatricians and parents as co-fiduciaries of pediatric patients In: Miller G, ed. Pediatric Bioethics. Cambridge, UK: Cambridge University Press; 2009:11–24. [Google Scholar]
- 89. Meller R, Willbold E, Hesse E, et al. Histologic and biomechanical analysis of anterior cruciate ligament graft to bone healing in skeletally immature sheep. Arthroscopy. 2008;24:1221–1231. [DOI] [PubMed] [Google Scholar]
- 90. Moksnes H, Engebretsen L, Eitzen I, et al. Functional outcomes following a non-operative treatment algorithm for anterior cruciate ligament injuries in skeletally immature children 12 years and younger: a prospective cohort with 2 years follow-up. Br J Sports Med. 2013;47:488–494. [DOI] [PubMed] [Google Scholar]
- 91. Moksnes H, Engebretsen L, Risberg MA. The current evidence for treatment of ACL injuries in children is low: a systematic review. J Bone Joint Surg Am. 2012;94:1112–1119. [DOI] [PubMed] [Google Scholar]
- 92. Moksnes H, Engebretsen L, Risberg MA. Prevalence and incidence of new meniscus and cartilage injuries after a nonoperative treatment algorithm for ACL tears in skeletally immature children. Am J Sports Med. 2013;41:1771–1779. [DOI] [PubMed] [Google Scholar]
- 93. Moksnes H, Engebretsen L, Seil R. The ESSKA paediatric anterior cruciate ligament monitoring initiative. Knee Surg Sports Traumatol Arthrosc. 2016;24:680–687. [DOI] [PubMed] [Google Scholar]
- 94. Murray MM. Current status and potential of primary ACL repair. Clin Sports Med. 2009;28:51–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Myklebust G, Engebretsen L, Braekken IH, et al. Prevention of anterior cruciate ligament injuries in female team handball players: a prospective intervention study over three seasons. Clin J Sport Med. 2003;13:71–78. [DOI] [PubMed] [Google Scholar]
- 96. Nair T, Savulescu J, Everett J, et al. Settling for second best: when should doctors agree to parental demands for suboptimal medical treatment? J Med Ethics. 2017;43:831–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Newman JT, Carry PM, Terhune EB, et al. Factors predictive of concomitant injuries among children and adolescents undergoing anterior cruciate ligament surgery. Am J Sports Med. 2015;43:282–288. [DOI] [PubMed] [Google Scholar]
- 98. Nwachukwu BU, McFeely ED, Nasreddine A, et al. Arthrofibrosis after anterior cruciate ligament reconstruction in children and adolescents. J Pediatric Orthop. 2011;31:811–817. [DOI] [PubMed] [Google Scholar]
- 99. Örtqvist M, Roos EM, Broström EW, et al. Development of the Knee injury and Osteoarthritis Outcome Score for Children (KOOS-Child): comprehensibility and content validity. Acta Orthop. 2012;83:666–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Paterno MV, Rauh MJ, Schmitt LC, et al. Incidence of second ACL injuries 2 years after primary ACL reconstruction and return to sport. Am J Sports Med. 2014;42:1567–1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Podlog L, Dimmock J, Miller J. A review of return to sport concerns following injury rehabilitation: practitioner strategies for enhancing recovery outcomes. Phys Ther Sport. 2011;12:36–42. [DOI] [PubMed] [Google Scholar]
- 102. Proffen BL, Fleming BC, Murray MM. Histologic predictors of maximum failure loads differ between the healing ACL and ACL grafts after 6 and 12 months in vivo. Orthop J Sports Med. 2013;1:2325967113512457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Pujol N, Beaufils P. Healing results of meniscal tears left in situ during anterior cruciate ligament reconstruction: a review of clinical studies. Knee Surg Sports Traumatol Arthrosc. 2009;17:396–401. [DOI] [PubMed] [Google Scholar]
- 104. Renström P, Johnson RJ. Anatomy and biomechanics of the menisci. Clin Sports Med. 1990;9:523–538. [PubMed] [Google Scholar]
- 105. Rhodes R, Holzman IR. The not unreasonable standard for assessment of surrogates and surrogate decisions. Theor Med Bioeth. 2004;25:367–386. [DOI] [PubMed] [Google Scholar]
- 106. Rössler R, Donath L, Bizzini M, et al. A new injury prevention programme for children’s football—FIFA 11+ Kids—can improve motor performance: a cluster-randomised controlled trial. J Sports Sci. 2016;34:549–556. [DOI] [PubMed] [Google Scholar]
- 107. Rössler R, Junge A, Bizzini M, et al. A multinational cluster randomised controlled trial to assess the efficacy of “11+ Kids”: a warm-up programme to prevent injuries in childrens’ football [published online December 22, 2017]. Sports Med. doi:10.1007/s40279-017-0834-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Scheffler SU, Schmidt T, Gangéy I, et al. Fresh-frozen free-tendon allografts versus autografts in anterior cruciate ligament reconstruction: delayed remodeling and inferior mechanical function during long-term healing in sheep. Arthroscopy. 2008;24:448–458. [DOI] [PubMed] [Google Scholar]
- 109. Schmidt LJ, Garratt AM, Fitzpatrick R. Child/parent-assessed population health outcome measures: a structured review. Child Care Health Dev. 2002;28:227–237. [DOI] [PubMed] [Google Scholar]
- 110. Seil R, Weitz F, Menetrey J, et al. Anatomical and technical considerations for pediatric ACL reconstruction In: Nakamura N, Zaffagnini S, Marx RG, Musahl V, eds. Controversies in the Technical Aspects of ACL Reconstruction. Heidelberg, Germany: Springer-Verlag; 2017:61–72. [Google Scholar]
- 111. Seil R, Weitz FK, Pape D. Surgical-experimental principles of anterior cruciate ligament (ACL) reconstruction with open growth plates. J Exp Orthop. 2015;2:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Shaw L, Finch CF. Trends in pediatric and adolescent anterior cruciate ligament injuries in Victoria, Australia 2005-2015. Int J Environ Res Public Health. 2017;14:599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Shieh AK, Edmonds EW, Pennock AT. Revision meniscal surgery in children and adolescents: risk factors and mechanisms for failure and subsequent management. Am J Sports Med. 2016;44:838–843. [DOI] [PubMed] [Google Scholar]
- 114. Siebold R, Takada T, Feil S, et al. Anatomical “C”-shaped double-bundle versus single-bundle anterior cruciate ligament reconstruction in pre-adolescent children with open growth plates. Knee Surg Sports Traumatol Arthrosc. 2016;24:796–806. [DOI] [PubMed] [Google Scholar]
- 115. Silvers-Granelli H, Mandelbaum B, Adeniji O, et al. Efficacy of the FIFA 11+ injury prevention program in the collegiate male soccer player. Am J Sports Med. 2015;43:2628–2637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Solans M, Pane S, Estrada MD, et al. Health-related quality of life measurement in children and adolescents: a systematic review of generic and disease-specific instruments. Value Health. 2008;11:742–764. [DOI] [PubMed] [Google Scholar]
- 117. Soligard T, Myklebust G, Steffen K, et al. Comprehensive warm-up programme to prevent injuries in young female footballers: cluster randomised controlled trial. BMJ. 2008;337:a2469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Soligard T, Nilstad A, Steffen K, et al. Compliance with a comprehensive warm-up programme to prevent injuries in youth football. Br J Sports Med. 2010;44:787–793. [DOI] [PubMed] [Google Scholar]
- 119. Steffen K, Emery CA, Romiti M, et al. High adherence to a neuromuscular injury prevention programme (FIFA 11+) improves functional balance and reduces injury risk in Canadian youth female football players: a cluster randomised trial. Br J Sports Med. 2013;47:794–802. [DOI] [PubMed] [Google Scholar]
- 120. Sugimoto D, Myer GD, McKeon JM, et al. Evaluation of the effectiveness of neuromuscular training to reduce anterior cruciate ligament injury in female athletes: a critical review of relative risk reduction and numbers-needed-to-treat analyses. Br J Sports Med. 2012;46:979–988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Tamin J. Models of occupational medicine practice: an approach to understanding moral conflict in “dual obligation” doctors. Med Health Care Philos. 2013;16:499–506. [DOI] [PubMed] [Google Scholar]
- 122. Tanner JM. Growth at Adolescence. Springfield, IL: Thomas; 1962. [Google Scholar]
- 123. Tejwani SG, Chen J, Funahashi TT, et al. Revision risk after allograft anterior cruciate ligament reconstruction. Am J Sports Med. 2015;42:2696–2705. [DOI] [PubMed] [Google Scholar]
- 124. Thapa MM, Chaturvedi A, Iyer RS, et al. MRI of pediatric patients: part 2, normal variants and abnormalities of the knee. AJR Am J Roentgenol. 2012;198:W456–W465. [DOI] [PubMed] [Google Scholar]
- 125. Thomeé R, Kaplan Y, Kvist J, et al. Muscle strength and hop performance criteria prior to return to sports after ACL reconstruction. Knee Surg Sports Traumatol Arthrosc. 2011;19:1798–1805. [DOI] [PubMed] [Google Scholar]
- 126. Thorborg K, Krommes KK, Esteve E, et al. Effect of specific exercise-based football injury prevention programmes on the overall injury rate in football: a systematic review and meta-analysis of the FIFA 11 and 11+ programmes. Br J Sports Med. 2017;51:562–571. [DOI] [PubMed] [Google Scholar]
- 127. van der Horst N, Backx FJG, Goedhart EA, et al. Return to play after hamstring injuries in football (soccer): a worldwide Delphi procedure regarding definition, medical criteria and decision-making. Br J Sports Med. 2017;51:1583–1591. [DOI] [PubMed] [Google Scholar]
- 128. van Melick N, van Cingel RE, Brooijmans F, et al. Evidence-based clinical practice update: practice guidelines for anterior cruciate ligament rehabilitation based on a systematic review and multidisciplinary consensus. Br J Sports Med. 2016;50:1506–1515. [DOI] [PubMed] [Google Scholar]
- 129. Varni JW, Seid M, Rode CA. The PedsQL: measurement model for the pediatric quality of life inventory. Med Care. 1999;37:126–139. [DOI] [PubMed] [Google Scholar]
- 130. Waldén M, Atroshi I, Magnusson H, et al. Prevention of acute knee injuries in adolescent female football players: cluster randomised controlled trial. BMJ. 2012;344:e3042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Wall J. Ethics in Light of Childhood. Washington, DC: Georgetown University Press; 2010. [Google Scholar]
- 132. Warme WJ, Mickelson D. All-epiphyseal semitendinosus PCL reconstruction in a 10-year-old child. J Pediatric Orthop. 2010;30:465–468. [DOI] [PubMed] [Google Scholar]
- 133. Werner BC, Yang S, Looney AM, et al. Trends in pediatric and adolescent anterior cruciate ligament injury and reconstruction. J Pediatric Orthop. 2015;36:447–452. [DOI] [PubMed] [Google Scholar]
- 134. Whittaker JL. Outcomes associated with early post-traumatic osteoarthritis and other negative health consequences 3-10 years following knee joint injury in youth sport. Osteoarthritis Cartilage. 2015;23:1122–1129. [DOI] [PubMed] [Google Scholar]
- 135. Wiggins AJ, Grandhi RK, Schneider DK, et al. Risk of secondary injury in younger athletes after anterior cruciate ligament reconstruction: a systematic review and meta-analysis. Am J Sports Med. 2016;44:1861–1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Williams BT, James EW, LaPrade RF. A physeal-sparing fibular collateral ligament and proximal tibiofibular joint reconstruction in a skeletally immature athlete. Knee Surg Sports Traumatol Arthrosc. 2016;24:661–665. [DOI] [PubMed] [Google Scholar]
- 137. Wyatt RW, Inacio MC, Liddle KD, et al. Factors associated with meniscus repair in patients undergoing anterior cruciate ligament reconstruction. Am J Sports Med. 2013;41:2766–2771. [DOI] [PubMed] [Google Scholar]
- 138. Yellin JL, Fabricant PD, Gornitzky A, et al. Rehabilitation following anterior cruciate ligament tears in children: a systematic review. JBJS Rev. 2016;4 doi:10.2106/JBJS.RVW.O.00001 [DOI] [PubMed] [Google Scholar]
- 139. Zebis MK, Andersen LL, Bencke J, et al. Identification of athletes at future risk of anterior cruciate ligament ruptures by neuromuscular screening. Am J Sports Med. 2009;37:1967–1973. [DOI] [PubMed] [Google Scholar]
- 140. Zebis MK, Bencke J, Andersen LL, et al. The effects of neuromuscular training on knee joint motor control during sidecutting in female elite soccer and handball players. Clin J Sport Med. 2008;18:329–337. [DOI] [PubMed] [Google Scholar]