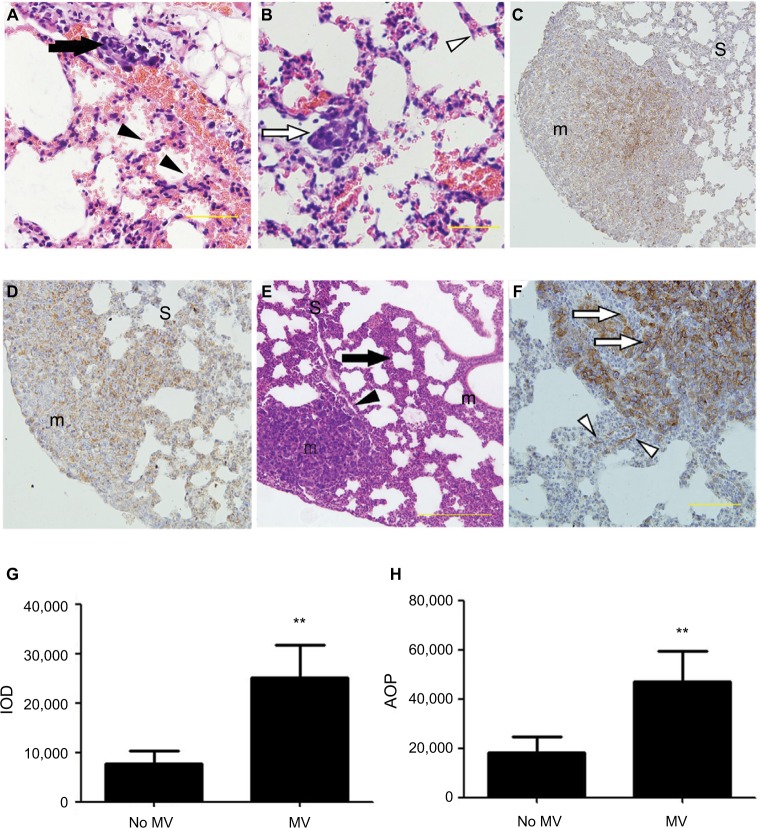
Figure 2.
(A–F) Representative histological view and images of immunohistochemical staining using anti-EpCAM body in the lung treated with MV or without MV. H&E stain of lung sections (A and B) shows micro-metastases formation and mild lung injury 24 h after intravenous 4T1 cells inoculation and MV treatment (Protocol A). Bar: 50 µm. (A) Arrow shows the presence of small-blood-vessel hemorrhaging and vascular congestion of tumor emboli. The majority of tumor cells were found lining the interior of blood vessels. However, a few tumor cells were found spreading into the surrounding tissue in the areas wherein blood vessel integrity was lost (indicated by an arrowhead). Note the presence of minimal interstitial edema and increase in intravascular and interstitial inflammatory cells in the same areas (indicated by an arrowhead). (B) Empty arrow shows the presence of a single tumor nodule within a lung parenchyma injury field (the “seeding” region) and empty arrowhead indicates normal lung parenchyma, whereas the lungs show intact blood vessels, bronchioles, and alveolar spaces. (C) Low power (×10) photomicrograph of anti-EpCAM immunohistochemical staining in lung tissue sections taken from BALB/c mice in the control group (28 d after 5×105 4T1 cells were injected into the mammary fat pad, untreated with MV during surgical procedures 14 d after inoculation [Protocol B]). The arrowhead indicates weak EpCAM immunohistochemical staining in 4T1 cells in the central part of a single tumor nodule. Intact surrounding lung parenchyma is negative for EpCAM. m, tumour nodule. (D) Treated MV during surgical procedures (Protocol B) shows that almost all 4T1 cells express EpCAM in a single tumor nodule. Weak-to-moderate EpCAM immunohistochemistry staining was observed in the surrounding lung parenchyma. H&E staining of lung sections (E) shows extensive tumor cell metastasis and lung architecture destruction. Arrows show diffuse increase of interstitial cellularity, with both mononuclear cells and neutrophilic infiltration; the arrowhead indicates blood vessel formation within the tumor nodule. Bar: 100 µm. (F) High-power (×40) photomicrograph of lung, 28 d after 5×105 4T1 cells were injected into the mammary fat pad, treated with MV during surgical procedures, and 14 d after inoculation (Protocol B), 4T1 tumor cells were observed with strong membrane or cytoplasmic staining of EpCAM within the tumor nodule (indicated by empty arrows) and a few epithelial cells with strong cytoplasmic EpCAM immunohistochemical staining were observed surrounding the tumor nodule (indicated by empty arrowhead). Bar: 50 µm. (G) Quantification of EpCAM with integrated optical density (IOD) in the lung metastatic tumor nodule of the MV exposure group and control group by immunohistochemical staining. Data presented as mean±SD, n=4; the ** indicates statistical significance, P<0.01. (H) Quantification of EpCAM with the area of positive staining (AOP) in lung metastatic tumor nodules in the MV exposure group and control group. Data presented as mean±SD, n=4; the ** indicates statistical significance, P<0.01.
Abbreviations: d, days; EpCAM, epithelial cell adhesion molecule; h, hours; m, tumor nodule; MV, mechanical ventilation; S, surrounding lung parenchyma.