Abstract
Arterial calcification reflects an atherosclerotic process associated with vascular stiffness. Whether baseline coronary artery calcium (CAC) and extra-coronary calcium (ECC), measured using noncontrast computed tomography imaging, are associated with incident hypertension is poorly understood. We studied participants from the Multi-Ethnic Study of Atherosclerosis without measured or self-reported hypertension at baseline. Incident hypertension was defined by blood pressure criteria (BP, ≥140/90 mmHg), BP medication use, or both, and was assessed at in-person visits. We analyzed incident hypertension using multivariable-adjusted discrete-time proportional hazards models. Net reclassification improvement (NRI) assessed whether CAC reclassified hypertension risk when added to the Framingham hypertension risk score. Among 3,304 subjects analyzed, mean age was 59 ± 10 years; 48% were male and 42% were white. There were 1,283 incident hypertension cases over a median (interquartile range) follow-up time of 10.6 (4.5, 11.5) years. Each 1-unit increase in ln(CAC+1) was independently associated with a 12% higher risk of hypertension (95% confidence interval [CI] 9% to 16%). Relative to CAC = 0, patients with CAC >400 had a hazard ratio for incident hypertension of 2.2 (95% CI 1.8 to 2.9). There was no interaction by age, gender, or baseline BP (p = 0.43, 0.19, 0.09, respectively). Continuous NRI analyses demonstrated that CAC can reclassify risk of incident hypertension; NRI = 0.19 (95% CI 0.10 to 0.26). Furthermore, all measurements of ECC were significantly associated with incident hypertension, even after adjustment for CAC (hazard ratios ranging from 1.36 to 1.38). In conclusion, patients with CAC and ECC are at markedly higher risk of incident hypertension and may benefit from more intensified prevention efforts.
Few studies have evaluated whether coronary artery calcium (CAC) can predict incident hypertension. In a previous study from the Multi-Ethnic Study of Atherosclerosis (MESA) (with follow-up through visit 4 in 2005 to 2007),1 CAC was found to have a significant association with incident hypertension in demographic-adjusted models only with no association found after adjustment for potential confounders. In another clinic-based study, CAC presence and severity at baseline were associated with incident hypertension in multivariable models that were adjusted for traditional risk factors.2 Neither study performed subgroup analyses, presented data on the association of temporal CAC changes with subsequent hypertension risk, evaluated the association between aortic and extra-coronary calcium (ECC) and hypertension, nor assessed the potential clinical utility of CAC for reclassification of hypertension risk estimates. Therefore, we sought to study the association between baseline CAC, CAC incidence and progression, and ECC with subsequent diagnosis of incident hypertension in MESA, using updated follow-up data through visit 5 (2010 to 2012) and performing comprehensive subgroup analyses by age, gender, race, and baseline blood pressure (BP) status.
Methods
MESA is a multiethnic, multicenter, prospective observational cohort study of 6,814 men and women aged 45 to 84 years without cardiovascular disease at baseline (participation rate was 60% among those eligible) who were recruited between July 2000 and August 2002 from 6 US communities (Baltimore, Maryland; Chicago, Illinois; Forsyth County, North Carolina; Los Angeles County, California; northern Manhattan, New York; and St. Paul, Minnesota). The cohort includes 42% whites (n = 1,404), 20% African-American (n = 662), 24% Hispanic (n = 792), and 14% Chinese (n = 446). Four follow-up visits were conducted in 2002 to 2004, 2004 to 2006, 2005 to 2007, and 2010 to 2012. There were 4,716 participants who attended the fifth MESA visit (31% withdrawal rate due to death or loss to follow-up by the final visit). All participants provided written informed consent, and the study was approved by the institutional review boards at all field centers.3 We excluded participants with prevalent or missing hypertension status according to BP values (n = 3,304), and those with self-reported hypertension (n = 206). Prevalent hypertension at baseline was defined as measured systolic BP ≥140 mmHg, diastolic BP ≥90 mmHg, or use of antihypertension medications.
Baseline CAC was assessed by noncontrast cardiac computed tomography (CT) using either cardiac-gated electron-beam CT or multidetector CT systems, depending on study site. Both scanner types yield near-identical results.4 A second CAC measurement was performed on 1/2 of the MESA cohort at visit 2, and the other half at visit 3, an average of 1.6 and 3.2 years after baseline (from 2002 to 2005). CAC incidence was defined as any detectable CAC among subjects without CAC at baseline (CAC = 0). CAC progression was defined as absolute increase in CAC score among participants with detectable baseline CAC (CAC >0).5
Aortic valve calcium was measured within the aortic valve cusps. Aortic root calcium was defined as calcified lesions in the aortic root at the level of the aortic ring. Thoracic aortic calcium was defined as calcification in the aortic wall above both the aortic root and the pulmonary trunk bifurcation, extending anywhere all the way up along the aortic arch and down the descending thoracic aorta to the level of the diaphragm.6,7
At each follow-up visit in the MESA study, systolic and diastolic BP measurements were recorded 3 times using a Dinamap Pro-100 automated oscillometric sphygmomanometer,8 and the mean of the last 2 measurements was used. Incident hypertension at each visit was defined based on BP criteria alone (systolic BP ≥140 mmHg or diastolic BP ≥90 mmHg), new use of antihypertensive medication alone, or both.
Information on demographic characteristics and cardiovascular risk factors was collected using standardized and validated questionnaires.3 Participants were asked to bring to the clinic containers for all medications used during the 2 weeks before the visit. Cigarette smoking and alcohol use were characterized as never, former, or current. Body mass index was calculated as weight in kilograms divided by height in meters squared. Diabetes mellitus was defined as a fasting blood glucose concentration of ≥126 mg/dl, self-reported diabetes, or the use of insulin or oral hypoglycemic medications. Fasting total cholesterol, high-density lipoprotein cholesterol, and triglyceride were measured at a central laboratory (University of Minnesota). Low-density lipoprotein cholesterol was calculated using the Friedewald equation. Family history of coronary heart disease was defined as history of a fatal or nonfatal myocardial infarction, coronary angioplasty, or coronary-artery bypass surgery in any first-degree relative regardless of age. Parental history of hypertension was defined as history of hypertension in a participant’s biologic mother or father. Estimated glomerular filtration rate was calculated using the Modification of Diet in Renal Disease (MDRD) equations. Physical activity was self-reported and healthy diet was evaluated using the Mediterranean-style diet score, which assesses participants’ consumption of heart-healthy or less healthy foods relative to the MESA population median.9
Baseline characteristics were stratified by incident hypertension status. Categorical variables were reported as percentages and compared using chi-square test, whereas continuous variables were reported using mean (standard deviation) or median (interquartile range) depending on normality of the data and compared using Student’s t test or Wilcoxon rank test, respectively. Unadjusted incidence rates of hypertension were calculated for the 4 baseline CAC categories (0, 1 to 100, 101 to 400, and >400)10–13 as number of events per 1,000 person-years. To describe the relation between baseline CAC as the exposure and hypertension as the outcome, we constructed Kaplan-Meier curves by CAC score category. We used complementary log-log models to derive hazard ratios (HRs) of incident hypertension according to CAC as both a continuous exposure variable (log normal [ln] CAC+1) and a categorical exposure variable. We tested for interaction of baseline CAC and age (≥60 years vs <60 years), gender, race/ethnicity, and baseline BP groups (normotension <120/80 mmHg vs prehypertension 120 to 139/80 to 90 mmHg) in the multivariable adjusted model (model 2), and stratified results where appropriate.
In analyses using CAC incidence as the exposure, we excluded incident hypertension cases that preceded the followup visit at which CAC >0 was detected. HRs of subsequent diagnosis of incident hypertension were calculated for those with incident CAC compared with those without. For CAC progression, we performed similar analyses using the absolute annualized difference in CAC score between baseline and follow-up as the exposure. In these analyses models were further adjusted for number of years between CAC scans. We additionally adjusted for baseline ln(CAC+1) among those with CAC >0 in analyses of CAC progression and also adjusted for baseline and mean change systolic and diastolic BP from visit 1 to visit 5.
Aortic valve calcium, aortic root calcium, and thoracic aortic calcium were categorized by presence (yes vs no) and by severity. In this latter analysis, we grouped ECC into 3 categories at each site: the first included those with Agatston score of 0, whereas the second and third were defined as less than or greater than the median score at each site among those with Agatston score >0. Model 3 in this analysis adjusted for Model 2 covariates plus baseline ln(CAC+1). Model 4 was adjusted for Model 3 covariates and all ECC measurements simultaneously. We also modeled ECC as an ordinal score according to number of ECC sites for aortic valve calcium, aortic root calcium, and thoracic aortic calcium.
The ability of CAC to reclassify risk of incident hypertension was assessed using continuous net reclassification improvement (NRI) and integrated discrimination index for the addition of CAC to the Framingham 4-year hypertension risk score and race/ethnicity.14,15 Risk estimates were calibrated to account for 10-year follow-up of hypertension in MESA by multiplying the estimates by 10 and dividing by 4.
To test the robustness of our results, we performed several sensitivity analyses: (1) We excluded hypertension cases defined solely on the basis of antihypertensive medication use; (2) We excluded subjects with borderline hypertension at baseline (systolic BP >130 mmHg or diastolic BP >85 mmHg); (3) We further adjusted for dietary salt intake; (4) We further adjusted for parental history of hypertension; (5) We further adjusted for estimated glomerular filtration rate and albuminuria; (6) We further adjusted for incident cardiovascular disease (CVD) cases, given CAC is associated with CVD and those who are diagnosed with CVD may be more likely to be diagnosed and treated as hypertensive; (7) We further adjusted for total cholesterol; (8) We studied the association between ECC and risk of incident hypertension among the subgroup of participants with CAC = 0 to determine the prognostic significance of ECC in the absence of coronary calcification; and (9) We accounted for competing risk from cardiovascular and non–cardiovascular-related causes of mortality using the method of Fine and Gray.16 Statistical significance was defined as p <0.05. Stata version 13.1 (Stata Corp, College Station, Texas) was used for all analyses.
Results
Participants who were excluded from this analysis on the basis of measured or self-reported hypertension at baseline differed from those who were included in our study (Supplementary Table S1). Our study sample consisted of 3,304 subjects free of hypertension at baseline. Baseline characteristics of the study sample are presented in Table 1. Compared with subjects who remained normotensive over follow-up, those who developed incident hypertension were more likely to be older, African-American, diabetic, use lipid-lowering medications, have a family history of coronary heart disease, have greater body mass index, former smokers, but less likely to have CAC = 0 at baseline (all p <0.05).
Table 1.
Baseline characteristics of the study population by incident hypertension status
| Variables | Overall (N = 3304) |
Not Hypertensive over follow up (N = 2021) |
Hypertensive over follow up (N = 1283) |
P-value |
|---|---|---|---|---|
| Age, (years) | 59 ± 10 | 57 ± 9 | 61 ± 10 | <0.001 |
| Males | 1592, 48% | 963, 48% | 629, 49% | 0.44 |
| White | 1404, 42% | 897, 44% | 507, 40% | <0.001 |
| Chinese | 446, 14% | 295, 15% | 151, 12% | |
| Black | 662, 20% | 340, 17% | 322, 25% | |
| Hispanic | 792, 24% | 489, 24% | 303, 24% | |
| Body mass index, (kg/m2) | 27.3 ± 5.1 | 26.6 ± 4.7 | 28.3 ± 5.4 | <0.001 |
| Diabetes mellitus | 207, 6% | 68, 3% | 139, 11% | <0.001 |
| Low-density lipoprotein cholesterol, (mg/dl) | 119 ± 31 | 120 ± 31 | 119 ± 31 | 0.50 |
| High-density lipoprotein cholesterol, (mg/dl) | 51 ± 15 | 52 ± 15 | 50 ± 15 | 0.01 |
| Lipid-lowering medication use | 326, 10% | 177, 9% | 149, 12% | 0.01 |
| Metabolic equivalents physical activity, min/week | 4560 (6199) | 4613 (6188) | 4413 (6248) | 0.32 |
| Healthy diet* | 1736, 53% | 1066, 53% | 670, 52% | 0.77 |
| Frequent addition of salt to food | 1392, 45% | 853, 45% | 539, 45% | 0.93 |
| Family history of CHD | 1210, 39% | 689, 36% | 521, 43% | <0.001 |
| Parental history of hypertension | 1268, 42% | 755, 42% | 513, 41% | 0.86 |
| eGFR, (ml/min/1.73 m2) | 83 ± 16 | 83 ± 15 | 84 ± 17 | 0.35 |
| Urinary albumin/creatinine (mg/g) | 4.3 ± 4.2 | 4.0 ± 3.5 | 4.9 ± 5.3 | <0.001 |
| Current cigarette smoking | 500, 15% | 313, 16% | 187, 15% | 0.049 |
| Current alcohol use | 1972, 60% | 1208, 60% | 764, 60% | 0.72 |
| Coronary artery calcium (CAC) | ||||
| CAC, Agatston units† | 0 (29) | 0 (15) | 0 (62) | <0.001 |
| CAC categories | <0.001 | |||
| CAC 0 | 2009 (61) | 1320 (65) | 689 (54) | |
| CAC 1–100 | 788 (24) | 449 (22) | 339 (26) | |
| CAC 101–400 | 329 (10) | 171 (8) | 158 (12) | |
| CAC >400 | 178 (5) | 81 (4) | 97 (8) | |
| Extra coronary calcium | ||||
| Aortic valve calcium (AVC), Agatston units† | 0 (0) | 0 (0) | 0 (0) | <0.001 |
| AVC categories | <0.001 | |||
| AVC 0 AU | 3044 (92) | 1898 (94) | 1146 (89) | |
| AVC 1–60 AU | 143 (4) | 70 (3) | 73 (6) | |
| AVC 61–7672 AU | 116 (4) | 52 (3) | 64 (5) | |
| Thoracic aortic calcium (TAC), Agatston units† | 0 (0) | 0 (0) | 0 (0) | <0.001 |
| TAC categories | <0.001 | |||
| TAC 0 AU | 2746 (83) | 1737 (86) | 1009 (79) | |
| TAC 1–267 AU | 328 (10) | 177 (9) | 151 (12) | |
| TAC 268–15163 AU | 229 (7) | 107 (5) | 122 (10) | |
| Aortic root calcium (ARC), Agatston units† | 0 (0) | 0 (0) | 0 (15) | <0.001 |
| ARC categories | <0.001 | |||
| ARC 0 AU | 2511 (76) | 1612 (80) | 899 (70) | |
| ARC 1–79 AU | 451 (14) | 240 (12) | 211 (16) | |
| ARC 80–3041 AU | 341 (10) | 168 (8) | 173 (13) |
Healthy diet consisted of adequate quantities of 5 items identified by the American Heart Association (fruits and vegetables, fish, whole grains, sodium < 1500 mg/day, and sugar-sweetened beverages ≤ 450 kcal (36 oz) per week).
Continuous variables are presented as mean (standard deviation) or median (interquartile range) and categorical variables as counts (percentage).
P-value calculated using Student’s t-test for normally distributed variables, Wilcoxon rank sum test for skewed variables, and chi-square test for categorical variables.
Bolded items are significant.
AU = Agatston Units; CHD = Coronary Heart Disease; eGFR = estimated glomerular filtration rate.
During a median interquartile range follow-up of 10.6 (4.5, 11.5) years, there were 1,283 incident cases of hypertension (Figure 1). We found a graded relation between increasing CAC score and risk of hypertension (Table 2 and Figure 2). In multivariable models, each 1 unit increase in ln(CAC+1) was associated with a 12% higher risk of incident hypertension (95% CI 9%, 16%). In categorical analyses, relative to CAC score of zero, CAC >400 was associated with a more than twofold increase in hypertension risk (HR 2.2, 95% CI 1.7, 2.9). Further adjustment for baseline systolic and diastolic BP yielded similar results (model 3). Interaction testing was significant only for race/ethnicity (p = 0.007). Stratified results by race/ethnicity were in general similar except for Chinese, where the association between CAC and incident hypertension was not significant (Supplementary Table S3). There was no interaction between CAC and age, gender, or baseline BP status (p = 0.43, 0.19, and 0.09, respectively).
Figure 1.
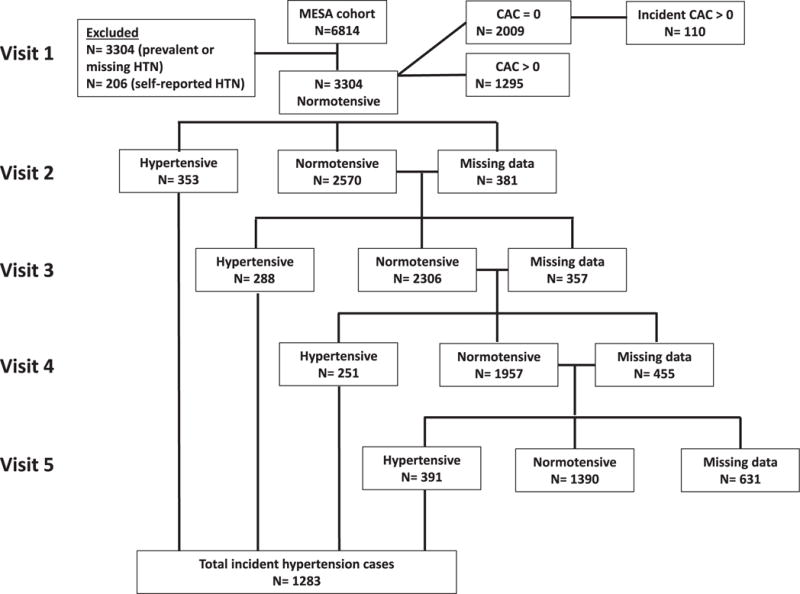
Incident hypertension status by MESA visit.
Table 2.
Hazard ratios (95% confidence interval) for the association of baseline coronary artery calcium and incident hypertension
| 1 unit continuous CAC* | CAC 0 | CAC 1–100 | CAC 101–400 | CAC >400 | p for trend | |
|---|---|---|---|---|---|---|
| N | − | 2009 | 788 | 329 | 178 | − |
| N incident HTN cases | − | 689 | 339 | 158 | 97 | − |
| Incidence Rates (per 1000 person-years) | − | 38.8 | 54.5 | 67.0 | 89.5 | − |
| Model 1 | 1.18 (1.15,1.21) | 1 (ref) | 1.50 (1.32,1.72) | 2.28 (1.90,2.74) | 3.21 (2.54,4.04) | <0.001 |
| Model 2 | 1.12 (1.09,1.16) | 1 (ref) | 1.35 (1.17,1.56) | 1.75 (1.44,2.13) | 2.24 (1.75,2.88) | <0.001 |
| Model 3 | 1.13 (1.10,1.16) | 1 (ref) | 1.40 (1.21,1.61) | 1.78 (1.47,2.17) | 2.24 (1.74,2.89) | <0.001 |
Modeled as ln(CAC+1).
Model 1 is adjusted for age, sex, race/ethnicity, education, MESA site.
Model 2 is adjusted for Model 1 covariates in addition to cigarette smoking, diabetes mellitus, low density lipoprotein cholesterol, high density lipoprotein cholesterol, cholesterol lowering medication use, family history of coronary heart disease, body mass index, moderate/vigorous physical activity, healthy diet, alcohol use.
Model 3 is adjusted for Model 2 covariates in addition to baseline systolic and diastolic blood pressure.
Figure 2.
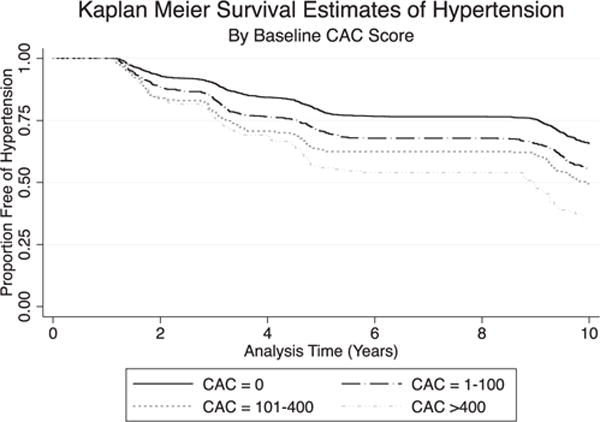
Kaplan-Meier survival estimates of incident hypertension by baseline coronary artery calcium score.
Only 110 cases of incident hypertension developed after the occurrence of incident CAC (baseline CAC = 0, follow-up CAC >0) (Figure 1). Despite this, in multivariable models assessing CAC incidence as the exposure and subsequent hypertension as the outcome, CAC incidence was associated with a 65% higher risk of incident hypertension (95% CI 3%, 260%). Among those with baseline CAC >0, there was a significant relation between absolute level of CAC progression and future hypertension risk, but this did not persist after accounting for baseline CAC. Further adjustment for baseline and mean interval change in systolic and diastolic BP yielded similar results (Supplementary Table S2).
There was a significant association of baseline presence of aortic valve calcium, aortic root calcium, and thoracic aortic calcium with incident hypertension after multivariable adjustment (inclusive of baseline CAC; Table 3). In analyses of ECC severity, there was a gradient for hypertension risk in demographic models, but this was attenuated after further adjustment for cardiovascular risk factors and baseline CAC. In general, these severity risk estimates were similar to ECC presence (yes/no) results. After accounting for all 3 measurements of ECC, thoracic aortic calcium and aortic root calcium remained independently associated with incident hypertension, while aortic valve calcium was no longer significant. In analyses of ECC ordinal score, there was a graded increase in risk of hypertension according to number of ECC sites (p trend <0.001) (Supplementary Table S4). Among those with CAC = 0, only aortic root calcium was significantly associated with hypertension risk (HR 1.71, 95% CI 1.36, 2.15).
Table 3.
Hazard ratios (95% confidence interval) for the association of extra coronary calcification and incident hypertension
| Aortic Valve Calcium (AVC) |
Thoracic Aortic Calcium (TAC) |
Aortic Root Calcium (ARC) |
||||
|---|---|---|---|---|---|---|
| Presence (Yes/No) |
By Severity (Categorical) |
Presence (Yes/No) |
By Severity (Categorical) |
Presence (Yes/No) |
By Severity (Categorical) |
|
| Model 1 | 2.09 (1.74,2.53) |
AVC 0 AU 1 (ref) |
2.13 (1.83,2.48) |
TAC 0 AU 1 (ref) |
2.01 (1.76,2.29) |
ARC 0 AU 1 (ref) |
| AVC 1–60 AU | TAC 1–267 AU | ARC 1–79 AU | ||||
| 1.91 (1.50,2.44) | 1.79 (1.49,2.15) | 1.78 (1.53,2.09) | ||||
| AVC 61–7672 AU | TAC 268–15163 AU | ARC 80–3041 AU | ||||
| 2.35 (1.80,3.06) | 2.91 (2.36,3.58) | 2.44 (2.04,2.91) | ||||
| Model 2 | 1.54 (1.26,1.88) |
AVC 0 AU 1 (ref) |
1.54 (1.31,1.82) |
TAC 0 AU 1 (ref) |
1.59 (1.38,1.83) |
ARC 0 AU 1 (ref) |
| AVC 1–60 AU | TAC 1–267 AU | ARC 1–79 AU | ||||
| 1.52 (1.18,1.95) | 1.43 (1.18,1.73) | 1.53 (1.29,1.81) | ||||
| AVC 61–7672 AU | TAC 268–15163 AU | ARC 80–3041 AU | ||||
| 1.57 (1.19,2.07) | 1.77 (1.41,2.22) | 1.68 (1.38,2.03) | ||||
| Model 3 | 1.36 (1.11,1.66) |
AVC 0 AU 1 (ref) |
1.36 (1.15,1.61) |
TAC 0 AU 1 (ref) |
1.38 (1.19,1.60) |
ARC 0 AU 1 (ref) |
| AVC 1–60 AU | TAC 1–267 AU | ARC 1–79 AU | ||||
| 1.31 (1.02,1.70) | 1.30 (1.07,1.58) | 1.38 (1.17,1.64) | ||||
| AVC 61–7672 AU | TAC 268–15163 AU | ARC 80–3041 AU | ||||
| 1.41 (1.07,1.86) | 1.46 (1.16,1.84) | 1.37 (1.12,1.67) | ||||
| Model 4 | 1.21 (0.99,1.49) |
AVC 0 AU 1 (ref) |
1.27 (1.07,1.50) |
TAC 0 AU 1 (ref) |
1.29 (1.11,1.51) |
ARC 0 AU 1 (ref) |
| AVC 1–60 AU | TAC 1–267 AU | ARC 1–79 AU | ||||
| 1.21 (0.92,1.56) | 1.24 (1.02,1.51) | 1.34 (1.13,1.59) | ||||
| AVC 61–7672 AU | TAC 268–15163 AU | ARC 80–3041 AU | ||||
| 1.29 (0.96,1.72) | 1.36 (1.07,1.73) | 1.19 (0.95,1.48) | ||||
AU = Agatston units.
Model 1 adjusted for age, sex, race/ethnicity, education, MESA site.
Model 2 adjusted for Model 1 covariates in addition to cigarette smoking, diabetes mellitus, low density lipoprotein cholesterol, high density lipoprotein cholesterol, cholesterol lowering medication use, family history of coronary heart disease, body mass index, moderate/vigorous physical activity, healthy diet, alcohol use, baseline CAC score.
Model 3 adjusted for Model 2 covariates in addition to baseline CAC score ln(CAC+1).
Model 4 is adjusted for Model 3 covariates in addition to all ECC measures simultaneously.
In continuous net reclassification improvement analyses, CAC reclassified risk of hypertension when added to the Framingham hypertension risk score and race/ethnicity; for example, a net reclassification improvement of 0.188 (95% CI 0.104, 0.263) for categorical CAC and 0.218 (95% CI 0.145,0.293) for continuous CAC. Integrated discrimination index analyses were not significant, however (Supplementary Table S5).
Analyses excluding incident hypertension defined based on antihypertensive medication use, further adjusting for salt intake, kidney disease, total cholesterol, family history of hypertension, or incident cardiovascular disease, and excluding participants with borderline hypertension yielded similar results. Only CAC >400 remained significantly associated with risk of hypertension after accounting for competing risk of cardiovascular-related mortality (HR 1.40, 95% CI 1.07, 1.83) and non–cardiovascular-related causes of mortality (HR 1.36, 95% CI 1.04, 1.79), respectively (Supplementary Table S3).
Discussion
In this multiethnic cohort, there was a graded association between baseline CAC and subsequent risk of incident hypertension. These results did not differ by age, gender, baseline BP status, but were not significant for Chinese ethnicity. The latter may be related to small size of the Chinese subgroup and race-stratified results were likely underpowered to detect significance in this group. In addition, CAC improved reclassification of hypertension risk in addition to known risk factors for hypertension. Incident development of CAC was also associated with subsequent risk of incident hypertension. Importantly, extra-coronary calcification, including aortic valve calcium, aortic root calcium, and thoracic aortic calcium, was also strongly associated with risk of hypertension, independent of baseline CAC score.
There are several potential explanations for the relation between CAC and incident hypertension. Subjects with elevated CAC typically carry a higher burden of cardiac risk factors, which are also associated with development of hypertension. Thus, the shared risk factors between CAC and hypertension may link both conditions (e.g., diabetes mellitus, low- or high-density lipoprotein cholesterol, and obesity).17 However, in our analysis, the association between CAC and hypertension persisted in models accounting for such variables.
CAC may also identify subjects with abnormal BP (either below the threshold for hypertension diagnosis or who have as yet to receive a diagnosis) complicated by endothelial dysfunction, aortic stiffness,18,19 and/or left ventricular hypertrophy,20–23 all of which are associated with hypertension risk. Furthermore, CAC may reflect the cumulative exposure of the vasculature to BP, including abnormal diurnal BP patterns, and masked hypertension, all of which increase susceptibility to development of hypertension but are not well-captured using office-based BP measurements.
Studies examining the relation between ECC and hypertension have, to date, been cross-sectional in nature.24 In MESA, Tison et al showed that the cross-sectional prevalence of hypertension increased with increasing number of ECC sites.25 We add to this work by demonstrating that ECC at baseline is prospectively associated with hypertension risk, independent of baseline CAC score. The mere presence of ECC appears to be just as predictive of hypertension risk as more complicated measurements of ECC severity, providing an opportunity for a more simplified application of this information to clinical practice. When the measurements of ECC were analyzed together, we found that calcification of the aorta itself, and not the aortic valve, was the main driver of the association between ECC and incident hypertension, a finding that is intuitive based on the association between vascular calcification and stiffness. The advantage of ECC is that it can be detected on a regular CAC scan and on CT imaging of chest (not cardiac-dedicated) done for screening/risk stratification purposes, requires no extra cost, and limits any additional radiation exposure.
Nonetheless, our results should also be interpreted in the context of several limitations. First, this study was observational, and therefore causality cannot be established. Second, CAC scores were made available to participants and their physicians. This may have altered prescription patterns of preventive pharmacotherapy and lifestyle habits among subjects with elevated CAC, which may have attenuated some of our associations. Third, although we attempted to adjust for many possible confounders in the relation between CAC and hypertension, there remains the possibility of residual confounding.26
In conclusion, we demonstrate that presence and severity of CAC score measured using cardiac CT scans not only identifies subjects who are at risk of hypertension, but also can reclassify risk of hypertension. In addition, extra-coronary calcification is associated with incident hypertension, over and above CAC burden. These inferences may also extend to CAC and ECC detected on non–cardiac-dedicated CT scans. Subjects with ECC or elevated CAC may therefore benefit from more frequent BP monitoring for timely diagnosis and treatment.
Supplementary Material
Acknowledgments
This research was supported by contracts HHSN268201500003I, N01-HC-95159, N01-HC-95160, N01-HC-95161, N01-HC-95162, N01-HC-95163, N01-HC-95164, N01-HC-95165, N01-HC-95166, N01-HC-95167, N01-HC-95168, and N01-HC-95169 from the National Heart, Lung, and Blood Institute and grants UL1-TR-000040 and UL1-TR-001079 from the National Center for Research Resources. Measurement of ECC was supported by R01 HL071739. The authors thank the other investigators, the staff, and the participants of the MESA study for their valuable contributions. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org.
Footnotes
See page 216 for disclosure information.
Disclosures:
Dr. Budoff serves on a speakers’ bureau for GE Healthcare. The remaining authors have no competing interests to declare.
Supplementary Data:
Supplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.amjcard.2017.10.018.
References
- 1.Peralta CA, Adeney KL, Shlipak MG, Jacobs D, Jr, Duprez D, Bluemke D, Polak J, Psaty B, Kestenbaum BR. Structural and functional vascular alterations and incident hypertension in normotensive adults: the Multi-Ethnic Study of Atherosclerosis. Am J Epidemiol. 2010;171:63–71. doi: 10.1093/aje/kwp319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grossman C, Shemesh J, Dovrish Z, Morag NK, Segev S, Grossman E. Coronary artery calcification is associated with the development of hypertension. Am J Hypertens. 2013;26:13–19. doi: 10.1093/ajh/hps028. [DOI] [PubMed] [Google Scholar]
- 3.Bild DE, Bluemke DA, Burke GL, Detrano R, Diez Roux AV, Folsom AR, Greenland P, Jacob DR, Jr, Kronmal R, Liu K, Nelson JC, O’Leary D, Saad MF, Shea S, Szklo M, Tracy RP. Multi-Ethnic Study of Atherosclerosis: objectives and design. Am J Epidemiol. 2002;156:871–881. doi: 10.1093/aje/kwf113. [DOI] [PubMed] [Google Scholar]
- 4.Carr JJ, Nelson JC, Wong ND, McNitt-Gray M, Arad Y, Jacobs DR, Jr, Sidney S, Bild DE, Williams OD, Detrano RC. Calcified coronary artery plaque measurement with cardiac CT in population-based studies: standardized protocol of Multi-Ethnic Study of Atherosclerosis (MESA) and Coronary Artery Risk Development in Young Adults (CARDIA) study. Radiology. 2005;234:35–43. doi: 10.1148/radiol.2341040439. [DOI] [PubMed] [Google Scholar]
- 5.Blaha MJ, DeFilippis AP, Rivera JJ, Budoff MJ, Blankstein R, Agatston A, Szklo M, Lakoski SG, Bertoni AG, Kronmal RA, Blumenthal RS, Nasir K. The relationship between insulin resistance and incidence and progression of coronary artery calcification: the Multi-Ethnic Study of Atherosclerosis (MESA) Diabetes Care. 2011;34:749–751. doi: 10.2337/dc10-1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Owens DS, Budoff MJ, Katz R, Takasu J, Shavelle DM, Carr JJ, Heckbert SR, Otto CM, Probstfield JL, Kronmal RA, O’Brien KD. Aortic valve calcium independently predicts coronary and cardiovascular events in a primary prevention population. JACC Cardiovasc Imaging. 2012;5:619–625. doi: 10.1016/j.jcmg.2011.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blaha MJ, Budoff MJ, Rivera JJ, Katz R, O’Leary DH, Polak JF, Takasu J, Blumenthal RS, Nasir K. Relationship of carotid distensibility and thoracic aorta calcification: Multi-Ethnic Study of Atherosclerosis. Hypertension. 2009;54:1408–1415. doi: 10.1161/HYPERTENSIONAHA.109.138396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chang JJ, Rabinowitz D, Shea S. Sources of variability in blood pressure measurement using the Dinamap PRO 100 automated oscillometric device. Am J Epidemiol. 2003;158:1218–1226. doi: 10.1093/aje/kwg274. [DOI] [PubMed] [Google Scholar]
- 9.Ahmed HM, Blaha MJ, Nasir K, Jones SR, Rivera JJ, Agatston A, Blankstein R, Wong ND, Lakoski S, Budoff MJ, Burke GL, Sibley CT, Ouyang P, Blumenthal RS. Low-risk lifestyle, coronary calcium, cardiovascular events, and mortality: results from MESA. Am J Epidemiol. 2013;178:12–21. doi: 10.1093/aje/kws453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Silverman MG, Harkness JR, Blankstein R, Budoff MJ, Agatston AS, Carr JJ, Lima JA, Blumenthal RS, Nasir K, Blaha MJ. Baseline subclinical atherosclerosis burden and distribution are associated with frequency and mode of future coronary revascularization: Multi-Ethnic Study of Atherosclerosis. JACC Cardiovasc Imaging. 2014;7:476–486. doi: 10.1016/j.jcmg.2014.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nasir K, Rubin J, Blaha MJ, Shaw LJ, Blankstein R, Rivera JJ, Khan AN, Berman D, Raggi P, Callister T, Rumberger JA, Min J, Jones SR, Blumenthal RS, Budoff MJ. Interplay of coronary artery calcification and traditional risk factors for the prediction of all-cause mortality in asymptomatic individuals. Circ Cardiovasc Imaging. 2012;5:467–473. doi: 10.1161/CIRCIMAGING.111.964528. [DOI] [PubMed] [Google Scholar]
- 12.Erbel R, Mohlenkamp S, Moebus S, Schmermund A, Lehmann N, Stang A, Dragano N, Grönemeyer D, Seibel R, Kälsch H, Bröcker-Preuss M, Mann K, Siegrist J, Jöckel KH. Coronary risk stratification, discrimination, and reclassification improvement based on quantification of subclinical coronary atherosclerosis. J Am Coll Cardiol. 2010;56:1397–1406. doi: 10.1016/j.jacc.2010.06.030. [DOI] [PubMed] [Google Scholar]
- 13.Vliegenthart R, Oudkerk M, Hofman A, Oei HH, van Dijck W, van Rooij FJ, Witteman JC. Coronary calcification improves cardiovascular risk prediction in the elderly. Circulation. 2005;112:572–577. doi: 10.1161/CIRCULATIONAHA.104.488916. [DOI] [PubMed] [Google Scholar]
- 14.Pencina MJ, D’Agostino RB, D’Agostino RB, Vasan RS. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat Med. 2008;27:157–172. doi: 10.1002/sim.2929. [DOI] [PubMed] [Google Scholar]
- 15.Parikh NI, Pencina MJ, Wang TJ, Benjamin EJ, Lanier KJ, Levy D, D’Agostino RB, Sr, Kannel WB, Vasan RS. A risk score for predicting near-term incidence of hypertension: the Framingham Heart Study. Ann Intern Med. 2008;148:102–110. doi: 10.7326/0003-4819-148-2-200801150-00005. [DOI] [PubMed] [Google Scholar]
- 16.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496–509. [Google Scholar]
- 17.Mahoney LT, Burns TL, Stanford W, Thompson BH, Witt JD, Rost CA, Lauer RM. Coronary risk factors measured in childhood and young adult life are associated with coronary artery calcification in young adults: the Muscatine Study. J Am Coll Cardiol. 1996;27:277–284. doi: 10.1016/0735-1097(95)00461-0. [DOI] [PubMed] [Google Scholar]
- 18.Tikhonoff V, Casiglia E. Measuring regional arterial stiffness in patients with peripheral artery disease: innovative technology. Hypertens Res. 2013;36:191–193. doi: 10.1038/hr.2012.178. [DOI] [PubMed] [Google Scholar]
- 19.Al-Mallah MH, Nasir K, Katz R, Takasu J, Lima JA, Bluemke DA, Hundley G, Blumenthal RS, Budoff MJ. Thoracic aortic distensibility and thoracic aortic calcium (from the Multi-Ethnic Study of Atherosclerosis [MESA]) Am J Cardiol. 2010;106:575–580. doi: 10.1016/j.amjcard.2010.03.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Post WS, Larson MG, Levy D. Impact of left ventricular structure on the incidence of hypertension. The Framingham Heart Study. Circulation. 1994;90:179–185. doi: 10.1161/01.cir.90.1.179. [DOI] [PubMed] [Google Scholar]
- 21.De Marco M, de Simone G, Roman MJ, Chinali M, Lee ET, Russell M, Howard BV, Devereux RB. Cardiovascular and metabolic predictors of progression of prehypertension into hypertension. Hypertension. 2009;54:974–980. doi: 10.1161/HYPERTENSIONAHA.109.129031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shimbo D, Muntner P, Mann D, Barr RG, Tang W, Post W, Lima J, Burke G, Bluemke D, Shea S. Association of left ventricular hypertrophy with incident hypertension: the Multi-Ethnic Study of Atherosclerosis. Am J Epidemiol. 2011;173:898–905. doi: 10.1093/aje/kwq509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McEvoy JW, Chen Y, Nambi V, Ballantyne CM, Sharrett AR, Appel LJ, Post WS, Blumenthal RS, Matsushita K, Selvin E. High-sensitivity cardiac troponin T and risk of hypertension. Circulation. 2015;132:825–833. doi: 10.1161/CIRCULATIONAHA.114.014364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tsao CW, Pencina KM, Massaro JM, Benjamin EJ, Levy D, Vasan RS, Hoffmann U, O’Donnell CJ, Mitchell GF. Cross-sectional relations of arterial stiffness, pressure pulsatility, wave reflection, and arterial calcification. Arterioscler Thromb Vasc Biol. 2014;34:2495–2500. doi: 10.1161/ATVBAHA.114.303916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tison GH, Guo M, Blaha MJ, McClelland RL, Allison MA, Szklo M, Wong ND, Blumenthal RS, Budoff MJ, Nasir K. Multisite extracoronary calcification indicates increased risk of coronary heart disease and all-cause mortality: the Multi-Ethnic Study of Atherosclerosis. J Cardiovasc Comput Tomogr. 2015;9:406–414. doi: 10.1016/j.jcct.2015.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McEvoy JW, Martin SS, Dardari ZA, Miedema MD, Sandfort V, Yeboah J, Budoff MJ, Goff DC, Jr, Psaty BM, Post WS, Nasir K, Blumenthal RS, Blaha MJ. Coronary artery calcium to guide a personalized risk-based approach to initiation and intensification of antihypertensive therapy. Circulation. 2017;135:153–165. doi: 10.1161/CIRCULATIONAHA.116.025471. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


