Abstract
Background:
The fat grafting process includes the 4 phases of tissue harvesting, processing, recipient-site preparation, and reinjection. Among them, the preparation of the recipient site has never been exhaustively reviewed. We aim to provide a comprehensive overview of the methods to prepare the recipient site through external expansion with the resulting outcomes.
Methods:
PubMed/Medline database was searched for studies on fat grafting recipient site preparation by applying the following algorithm: ((fat grafting) OR (lipofilling) OR (lipograft) AND (recipient site)). A priori criteria were used to review the resulting articles and identify those dealing with external expansion.
Results:
Fourteen studies published from 2008 through 2016 met inclusion criteria (4 case reports, 6 retrospective, and 4 prospective studies), representing 1,274 treated patients. Two devices for preexpansion were used with different protocols: BRAVA system and Kiwi VAC-6000M with a PalmPump. The 13 studies that applied the BRAVA system reported large fat volume transplantation to the breast (average > 200 cc). The most common complications were localized edema (14.2%), temporary bruising, and superficial skin blisters (11.3%), while the most serious was pneumothorax (0.5%). The majority of the studies reported enhancement of fat graft survival, which ranged between 53% and 82% at 6 months to 1 year follow-up, and high satisfaction of patients and surgeon.
Conclusions:
External expansion and fat grafting is a promising technique for breast reconstruction and augmentation. However, due to the overall low level of evidence of the available studies, further research is needed to validate the procedure.
INTRODUCTION
During the past decades, autologous fat grafting (AFG) has become a well-established procedure in Plastic Surgery, widely used for both reconstructive and aesthetic purposes.1–3 According to data released by the International Society of Aesthetic Plastic Surgery, it is indeed 1 of the most common operations for breast and buttock augmentation and facial rejuvenation, accounting for more than 1,000,000 procedures performed in 2016 over a total of 10,000,000.4 AFG is appreciated for providing an abundant and easily available source of tissue removed from a donor site with excessive unpleasant accumulation to a recipient site in need for volume enhancement. In addition, the proven regenerative potential expressed by its stromal vascular fraction, has been applied for the treatment of scars, scar-related conditions and burns.5,6
Notably, recent research has especially focused on 3 of the 4 phases of the procedure, namely fat harvesting, processing, and reinjection, while the additional step of recipient-site preparation has mainly been neglected.1,3 In particular, harvesting, processing, and reinjection were extensively examined in a recent comprehensive review by Strong et al.3 published in 2015, which is the most up-to-date available information on AFG.
Conversely, although many considerations were dedicated to the recipient-site preparation and great interest in this regard has been generated by the external expansion techniques, including the use of BRAVA system (Brava LLC, Miami, Fla.),7 this was never comprehensively or systematically reviewed. However, inter alia, several variables related to the recipient site in itself were already identified and correlated to AFG success (age of the patient, mobile versus less mobile areas of the face, trauma, burns, scars, structural defects, compartments on the face).8–10
The seek for evidence in fat grafting is motivated by the desire of establishing an ideal approach, which may guarantee optimal outcomes by understanding the reasons underling the current huge variability in terms of graft survival (30–80%) observed by different authors who used different methods.1 The aim of the present research is to present a comprehensive analysis of the international literature regarding all of the studies, which investigated recipient-site preparation with a focus on external expansion.
MATERIALS AND METHODS
Between May and June 2017, a literature review of the entire PubMed/Medline database was conducted to assess the efficacy and complications of AFG recipient-site preparation with external expansion. The search algorithm was: ((fat grafting) OR (lipofilling) OR (lipograft) AND (recipient site)).
Inclusion criteria were (1) clinical studies (case reports, retrospective or prospective case series, clinical trials); (2) application of a recipient-site external expansion technique before fat grafting. Excluded from the analysis were literature reviews and descriptive articles with no measurable endpoint.
No restrictions on time or language of publication were applied. References of the publications identified initially were screened to add studies fulfilling inclusion criteria.
All articles were screened manually. Two investigators (C.M.O. and J.S.) independently reviewed and extracted data from the publications, which were examined by a third reviewer (M.T.) in case of disagreement.
All types of external expansion techniques were considered. We documented and tabulated the following information for each article: author name(s), year of publication, external expansion procedure, study design, number of patients, indication for treatment, comparator, and outcomes/findings.
RESULTS
One hundred seventeen full-text articles were initially identified, 110 of which were excluded according to predetermined criteria. Seven articles were included after reviewing references of the publications identified initially. Therefore, our analysis comprised 14 studies, which were published from 2008 through 2016. Fourteen clinical studies on external expansion were performed on 1,274 patients (4 case reports, 6 retrospective, and 4 prospective studies). The maximum level of evidence was found to be equal to 3 in prospective case series. Surgical indications for fat grafting were breast reconstruction after treatment for cancer, breast augmentation for aesthetic purposes, correction of iatrogenic deformities (deformity after excision of a congenital nevus as child and deformity due to a surgical cardiac procedure), correction of congenital deformities, and the wish to replace preexisting implants. A detailed analysis of all studies is reported in Table 1.
Table 1.
Overview of the Studies Investigating Recipient Site External Expansion before Fat Grafting
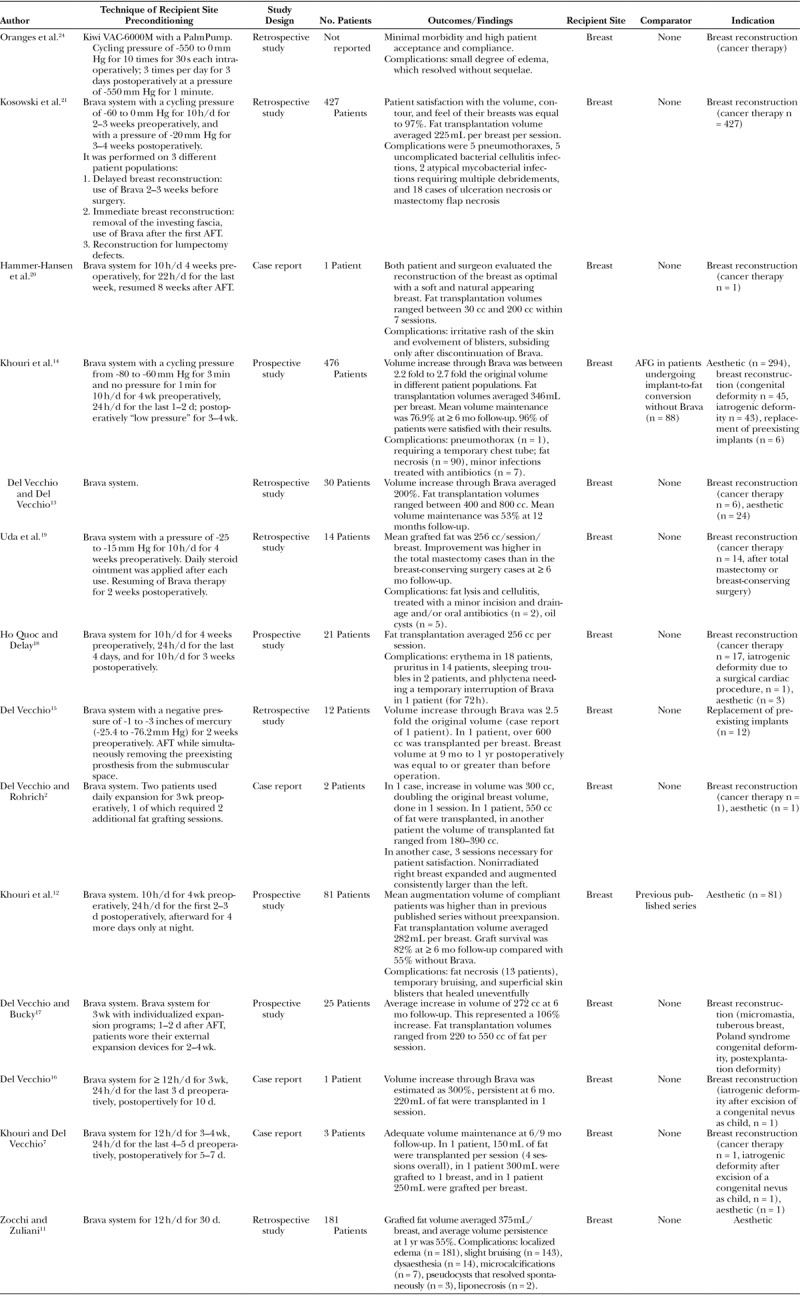
Thirteen authors used the Brava system (Brava LLC, Miami, Fla.), a bra-like device generating a low negative pressure (maximum, -80 mm Hg) worn for 10–24 h/d for 2–4 weeks preoperativly.2,7,11–21 Large amount of fat transplant was allowed, with 7 of the authors who performed megavolume fat transplant (≥ 300 cc)2,7,11,13–15,17 and almost the totality of cases (n = 1,272) receiving more than 200 cc of fat graft per session in average. One to 7 sessions were performed to achieve satisfactory results. The studies reported a mean fat graft survival ranging between 53% and 82%.11–14 Three studies, 2 of which compared their outcomes with previous published series, reported significant enhancement of fat graft survival in comparison with fat grafting without preexpansion.11,12,14
Brava system was used with a wide range of pressure values. Although some of the studies did not explicitly report on the pressure applied, we hypothesize that they used the device as initially described by Khouri et al.12,18–20,22,23 (-15 to -25 mm Hg). Kosowski et al.21 used a pressure cycling between -60 and 0 mm Hg preoperatively and -20 mm Hg postoperatively, whereas Khouri et al.14 in 2014 applied a pressure cycling between -80 and -60 mm Hg and “low pressure” postoperatively. Finally, Del Vecchio and Bucky17 stated that expansion programs were individualized for each patient based on lifestyle analysis and psychological compliance testing, with a negative pressure, which in 1 of his studies was ranging from -1 to -3 inches of mercury (-25.4 to -76.2 mm Hg).15
Four studies reported the use of cyclical pressure,14,18,21,24 whereas the remaining studies did not report on whether the device was used with continuous or cycling power, yet we believe that it was continuous as initially described.22
Regarding different time and durations of preexpansion, patients started their treatment with the external expansion device up to 4 weeks before autologous fat transfer for 10–24 h/d.2,7,11,12,14–19 Postoperatively, the Brava system was worn for 5 days to 4 weeks, with the duration of application ranging from 10 to 24 h/d, only at night or for as many hours per day as tolerated.
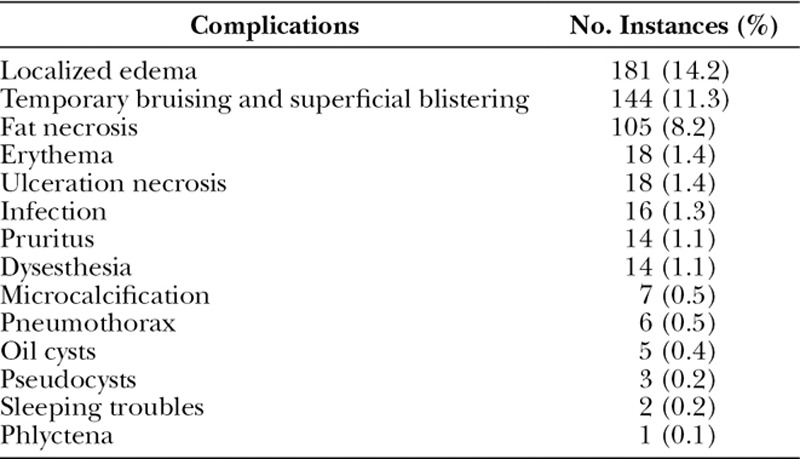
The most common complications using Brava system were localized edema (14.2%),11 temporary bruising and superficial skin blisters (11.3%),11,20 and fat necrosis (8.2%).11,12,14 The most serious complication was pneumothorax, which occurred in 6 cases (0.5%), 1 of which required chest tube.14,21 A complete list of complications observed with Brava system and AFG is reported in Table 2.
Table 2.
Complications Observed with the Use of Brava System and Fat Grafting
In 1 article from our group, it was used a smaller device called Kiwi VAC-6000M with a PalmPump (Clinical Innovations, South Murray, Utah), a complete vacuum delivery system, which applies a stronger cycling negative pressure (-550 mm Hg) for a much shorter intraoperative period (10 times for 30 seconds each) on localized scarred recipient sites before autologous fat injection.24 Postoperatively, the Kiwi VAC was applied 3 times per day for 1 minute each for 3 days. The authors reported a gross expansion of tissue, with a macroscopic swelling that regressed slowly after the end of the stimulation, and a small degree of edema, which resolved without sequelae as complication. They also observed satisfactory clinical outcomes, with minimal morbidity and high patient acceptance and compliance.
DISCUSSION
The preparation of the recipient site was recently reported as extremely relevant and commonly performed by surgeons treating contracted scar tissues.25,26 This aspect was extensively examined by Khouri et al.25 in a publication appeared in 2014 in this journal, which presented a comprehensive overview of fat grafting practice and techniques.26 Accordingly, the preparation of the recipient site was endorsed for the treatment of either contracted scar tissue or congenital constriction bands to allow a “cicatrix-to-matrix” transformation, with a release of contracture at the time of fat grafting.25,26
However, our review is the first that comprehensively reviewed the studies investigating the outcomes achieved with preexpansion performed for different indications and with different protocols. With our inclusion criteria, we identified 14 clinical articles describing the use of 2 external expansion devices, namely Brava system and Kiwi VAC-6000M with a PalmPump, 13 of which reported the treatment of 1,274 patients with Brava system.
The mechanism of action of preexpansion was investigated by 7 preclinical studies, which analyzed the impact of 4 variables: the value of the negative pressure applied, the strength of cycling versus static pressures, the duration and the timing of the preexpansion.
Four authors used a negative pressure of -25 mm Hg, equal to the most commonly used with Brava, in a mice model, observing tissue stretch, edema, and inflammation, factors which triggered cell proliferation, neoangiogenesis, and neoadipogenesis.24,27–30 Similarly, with a higher pressure (-70 mm Hg) applied to the dorsum of pigs, Hsiao et al.31 showed an increase in vascularity, cell proliferation, hair follicles number, and skin thickness, yet observing simultaneous skin loosening. Finally, Lee et al.32 applied a pressure of -125 mm Hg to the dorsal ear of 20 white rabbits for 1 week before fat grafting with the rationale of using the same negative pressure applied for noninvasive wound closure.33 They reported increased vascularization of the preexpanded recipient site and, accordingly, enhanced fat graft survival.32,33
Regarding the comparison between cyclical and static pressures, Chin et al.34 demonstrated that cyclical use of negative pressure provides a more robust response in terms of epidermal proliferation and angiogenesis.32
Finally, in relation to different duration and timing of exposure, in animal studies this ranged from 1 single application of 1.5 hours to 24 hours for 28 consecutive days.27–33 Notably, the animal study by Lujan-Hernandez et al.30 showed no significant difference in terms of neo-adipogenesis between tissues exposed to single 2 hours stimulation or to 2 hours daily for 5 days.33
Brava was initially described for nonsurgical breast augmentation by Khouri et al.22 in 2000 to exploit the ability of tissues to grow when subjected to controlled distractive mechanical forces. The patients of this initial study were asked to wear a brassiere-like system with 20 mm Hg vacuum distraction force to each breast for 10–12 h/d over a 10-week period, achieving 98 ± 67% average increase of the breast volume at the end of the expansion treatment, and 55% (range, 15–115%) at 30 weeks. The authors also reported very high patient satisfaction, no adverse events, and described the device as comfortable to wear.
However, after the enthusiasm generated by this first investigations, following researches outlined the limitations of the procedure: only small breast-size enlargement (1 cup) possible, high patient compliance required, patient social life restriction and drop out rates around 25%, 50% of the volume increase only due to swelling at 10 weeks with the suggestion to wear the device for 16–20 weeks.12,22,35–38
Despite the consequent modest success as nonsurgical breast augmentation procedure, the ability of Brava to determine a marked temporary increase in breast size with the creation of a very large fibrovascular scaffold induced several authors to investigate its potential as device to prepare the recipient site in fat grafting procedures.12 These studies reduced the duration of the original protocol, with external volume expansion evolving from 2 to 3 months of static low pressure to 3 weeks individualized programs.17
Del Vecchio and Bucky17 reported 5 main reasons supporting the use of Brava before fat grafting: (1) creation of more overall parenchymal space; (2) reduction of interstitial pressure in the breast for a given volume of transplanted graft; (3) modification of breast shape through augmentation of contour irregularities before grafting; (4) possibility to avoid variables such as high-speed centrifugation with resulting shorter operating room times; (5) angiogenesis, consequence of micromechanical forces on the recipient site.
Del Vecchio and Bucky17 also noted that the degree of physical expansion obtained with Brava is not merely depending on the compliance of the patient, but also on the mechanical compliance of the recipient site. Indeed, they reported that multiparous breasts expanded better than dense nulliparous breast, while constricted breasts expanded well but required additional treatments such as nipple-areola reductions, percutaneous release of constriction bands to lower the inframammary folds, or additional grafting session to reshape the breasts. Moreover, Kosowski et al.21 observed that irradiated tissue required more sessions of fat transplantation in comparison to nonradiated tissue to achieve satisfactory results, whereas Uda et al.19 observed little skin extension in irradiated breast-conserving cases, resulting in poor cosmetic scores. Finally, regarding recipient sites characterized by contracted scar tissue, Uda et al.19 reported higher improvement in case of total mastectomy compared with breast-conserving surgery, also in terms of total aesthetic score.
Recipient site features did not only impact the degree of expansion but also the complication rate. It was indeed observed a higher rate of complications in case of skin-sparing and nipple-sparing mastectomies, explained with the difficult release of folds and adhesions to the chest wall of mastectomy skin flaps and skin excess.21 A high rate of skin complications was also reported in case of use of Brava on irradiated breast, as radiation therapy causes thinning of the epithelial tissue, affects blood circulation in the dermal tissue, and decreases dermal appendages, therefore inhibiting the regenerative ability of the skin.19,39 Specifically, the ulceration rate was significantly larger in radiated breasts (6.5%) than in nonradiated breasts (1.4%) for postmastectomy patients in the study by Kosowski et al.21
However, although Uda et al.19 discouraged the use of Brava on irradiated tissue for the above-mentioned reasons, Kosowski et al.21 endorsed its use postulating that fat grafting to the irradiated breast could reverse radiation damage to yield superior results.40 Yet, also Kosowski et al.21 outlined that radiated breast tissue is less compliant, with consequent overgrafting and its inherent complications more likely to occur, recommending a greater craftsmanship and experience for a safe and effective execution of the procedure, and performance of multiple treatments (> 4) with small volumes of fat grafting.21
Notably, Hammer-Hansen et al.20 emphasized the relevance of the dermatological side effects of Brava. They urged studies documenting the extent of this problem to provide clinical guidance for future use of the device and thus minimize or completely avoid these complications in future patients. Our review identified and quantified skin side effects of Brava as follows: temporal bruising and superficial blistering, 11.3%; erythema, 1.4%; ulceration necrosis, 1.4%; pruritus, 1.1%; phlyctena, 0.1%.11,18,20,21
Pneumothorax, the most serious complication observed, was reported by 2 authors who analyzed 427 and 476 patients, respectively, and affected 6 patients (overall, 0.5%), one of which required chest tube.14,21
Among the advantages of Brava, the authors emphasized that preexpanded breasts accept a greater fat transplant volume per session with a higher retention rate, resulting in less necessary session to achieve satisfactory results.14,21 Indeed, we found 3 papers reporting significant enhancement of fat graft survival compared with fat grafting without preexpansion,11,12,14 leading Khouri et al.12 to state that if Brava is not used, the patient should accept smaller volume of fat grafting and multiple sessions, or life time implants in the aesthetic setting. We found a mean fat graft survival ranging between 53% and 82%.11–14 In this regard, although we believe that the most meaningful outcome measure in fat grafting is percent augmentation instead of percent survival, as suggested by Khouri and Khouri41 in 2015, we here refer to percent survival as this was the variable reported in the studies included in this review. The large amount of fat transplant possible after the use of Brava allowed total breast reconstruction and breast augmentation with satisfactory results. In fact, 7 authors performed megavolume fat transplant (≥ 300 cc),2,7,11,13–15,17 and almost all the patients (1,272 over 1,274) received an average of more than 200 cc of fat graft per session.
Moreover, Kosowski et al.21 observed that in contrast to traditional methods (implants and flaps), the use of Brava and autologuous fat transplantation holds the benefit to preserve or restore sensation of the breasts. However, our review also observed a rate of dysesthesia equal to 1.1%.11
Finally, our group described the use of an alternative device named Kiwi VAC-6000M with a PalmPump, for specific indications.24 This was reported as a simple intraoperative external expansion system, which applies the cycling negative pressure of -550 mm Hg for 5 minutes to enhance small-volume AFG (40–80 mL). The rationale of preparing the recipient site with Kiwi is to obtain the release of contracted scar tissues by exploiting its traction force, and to promote intense edema, ischemia, and inflammation providing an ideal environment for cell proliferation and angiogenesis. The technique was described as especially useful in case of restrictive subdermal cicatrix, through the creation of a vascularized scaffold that is seeded with fat grafts, and in case of retractions or scarring as a result of radiation therapy.
CONCLUSIONS
Overall, positive outcomes were demonstrated with the use of external expansion in all the articles identified for both aesthetic and reconstructive purposes. Indeed, by allowing megavolume fat transplantation (> 300 cc), it appeared to be a valid alternative to implants for breast augmentation, and to free flaps and implants for breast reconstruction after total mastectomy. However, the low level of evidence of the studies that was found to be equal to maximum 3 in prospective case series, demonstrated the need of further exploring this topic.
Footnotes
This work has been presented in part at the 53rd Annual Meeting of the Swiss Society of Plastic, Reconstructive and Aesthetic Surgery, September 1st through 2nd, 2017, St. Moritz, Switzerland; the 66th National Congress of the Italian Society of Plastic, Reconstructive and Aesthetic Surgery—1st Joint Meeting with the Brazilian Society of Plastic Surgery, in Modena, Italy, September 21st through 23rd, 2017; the 6th Annual Meeting of the International Society of Plastic & Regenerative Surgeons, November 17th through 19th, 2017, Dubai, United Arab Emirates; the 15th Annual Meeting of the International Federation for Adipose Therapeutics and Science, November 30th through December 3rd, 2017, Miami, Fla.; and the 9th International Conference on Regenerative Surgery, December 15th through 16th, 2017, Rome, Italy.
Dr. Oranges and J. Striebel contributed equally to this work.
Disclosure: The authors have no financial interest to declare in relation to the content of this article. The Article Processing Charge was paid for by the authors.
REFERENCES
- 1.Gir P, Brown SA, Oni G, et al. Fat grafting: evidence-based review on autologous fat harvesting, processing, reinjection, and storage. Plast Reconstr Surg. 2012;130:249–258.. [DOI] [PubMed] [Google Scholar]
- 2.Del Vecchio D, Rohrich RJ. A classification of clinical fat grafting: different problems, different solutions. Plast Reconstr Surg. 2012;130:511–522.. [DOI] [PubMed] [Google Scholar]
- 3.Strong AL, Cederna PS, Rubin JP, et al. The current state of fat grafting: a review of harvesting, processing, and injection techniques. Plast Reconstr Surg. 2015;136:897–912.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.International Society of Aesthetic Plastic Surgery (ISAPS). The international study on aesthetic/cosmetic procedures performed in 2016. Available at http://www.isaps.org/Media/Default/Current%20News/GlobalStatistics2016.pdf. Accessed October 5, 2017.
- 5.Negenborn VL, Groen JW, Smit JM, et al. The use of autologous fat grafting for treatment of scar tissue and scar-related conditions: a systematic review. Plast Reconstr Surg. 2016;137:31e–43e.. [DOI] [PubMed] [Google Scholar]
- 6.Condé-Green A, Marano AA, Lee ES, et al. Fat grafting and adipose-derived regenerative cells in burn wound healing and scarring: a systematic review of the literature. Plast Reconstr Surg. 2016;137:302–312.. [DOI] [PubMed] [Google Scholar]
- 7.Khouri R, Del Vecchio D. Breast reconstruction and augmentation using pre-expansion and autologous fat transplantation. Clin Plast Surg. 2009;36:269–280, viii.. [DOI] [PubMed] [Google Scholar]
- 8.Rohrich RJ. Ethical approval of clinical studies, informed consent, and the Declaration of Helsinki: what you need to know. Plast Reconstr Surg. 2007;119:2307–2309.. [DOI] [PubMed] [Google Scholar]
- 9.Wang W, Xie Y, Huang RL, et al. Facial contouring by targeted restoration of facial fat compartment volume: the midface. Plast Reconstr Surg. 2017;139:563–572.. [DOI] [PubMed] [Google Scholar]
- 10.Oranges CM, Tremp M, Haug M, et al. Facial contouring by targeted restoration of facial fat compartment volume: the midface. Plast Reconstr Surg. 2017;140:622e. [DOI] [PubMed] [Google Scholar]
- 11.Zocchi ML, Zuliani F. Bicompartmental breast lipostructuring. Aesthetic Plast Surg. 2008;32:313–328.. [DOI] [PubMed] [Google Scholar]
- 12.Khouri RK, Eisenmann-Klein M, Cardoso E, et al. Brava and autologous fat transfer is a safe and effective breast augmentation alternative: results of a 6-year, 81-patient, prospective multicenter study. Plast Reconstr Surg. 2012;129:1173–1187.. [DOI] [PubMed] [Google Scholar]
- 13.Del Vecchio DA, Del Vecchio SJ. The graft-to-capacity ratio: volumetric planning in large-volume fat transplantation. Plast Reconstr Surg. 2014;133:561–569.. [DOI] [PubMed] [Google Scholar]
- 14.Khouri RK, Khouri RK, Jr, Rigotti G, et al. Aesthetic applications of Brava-assisted megavolume fat grafting to the breasts: a 9-year, 476-patient, multicenter experience. Plast Reconstr Surg. 2014;133:796–807.; discussion 808–9. [DOI] [PubMed] [Google Scholar]
- 15.Del Vecchio DA. “SIEF”—simultaneous implant exchange with fat: a new option in revision breast implant surgery. Plast Reconstr Surg. 2012;130:1187–1196.. [DOI] [PubMed] [Google Scholar]
- 16.Del Vecchio D. Breast reconstruction for breast asymmetry using recipient site pre-expansion and autologous fat grafting: a case report. Ann Plast Surg. 2009;62:523–527.. [DOI] [PubMed] [Google Scholar]
- 17.Del Vecchio DA, Bucky LP. Breast augmentation using preexpansion and autologous fat transplantation: a clinical radiographic study. Plast Reconstr Surg. 2011;127:2441–2450.. [DOI] [PubMed] [Google Scholar]
- 18.Ho Quoc C, Delay E. [Tolerance of pre-expansion BRAVA and fat grafting into the breast]. Ann Chir Plast Esthet. 2013;58:216–221.. [DOI] [PubMed] [Google Scholar]
- 19.Uda H, Sugawara Y, Sarukawa S, et al. Brava and autologous fat grafting for breast reconstruction after cancer surgery. Plast Reconstr Surg. 2014;133:203–213.. [DOI] [PubMed] [Google Scholar]
- 20.Hammer-Hansen N, Jensen TB, Damsgaard TE. Delayed total breast reconstruction with Brava. Case Rep Surg. 2015;2015:601904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kosowski TR, Rigotti G, Khouri RK. Tissue-engineered autologous breast regeneration with Brava®-assisted fat grafting. Clin Plast Surg. 2015;42:325–37, viii.. [DOI] [PubMed] [Google Scholar]
- 22.Khouri RK, Schlenz I, Murphy BJ, et al. Nonsurgical breast enlargement using an external soft-tissue expansion system. Plast Reconstr Surg. 2000;105:2500–2512.; discussion 2513. [DOI] [PubMed] [Google Scholar]
- 23.Khouri RK, Del Vecchio D. Spear SL, Willey SC, Robb GL. Breast reconstruction and augmentation using BRAVA external breast expansion and autologous fat grafting. In: Surgery of the Breast: Principles and Art. 2011;Philadelphia, Pa: Wolters Kluwer/Lippincott Williams & Wilkins; 1374–1409.. [Google Scholar]
- 24.Oranges CM, Tremp M, Ling B, et al. A simple, reliable, and inexpensive intraoperative external expansion system for enhanced autologous structural fat grafting. Arch Plast Surg. 2016;43:466–469.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Khouri RK, Rigotti G, Cardoso E, et al. Megavolume autologous fat transfer: part II. Practice and techniques. Plast Reconstr Surg. 2014;133:1369–1377.. [DOI] [PubMed] [Google Scholar]
- 26.Oranges CM, Schaefer DJ. The use of autologous fat grafting for treatment of scar tissue and scar-related conditions: a systematic review. Plast Reconstr Surg. 2016;138: 551e–552e.. [DOI] [PubMed] [Google Scholar]
- 27.Heit YI, Lancerotto L, Mesteri I, et al. External volume expansion increases subcutaneous thickness, cell proliferation, and vascular remodeling in a murine model. Plast Reconstr Surg. 2012;130:541–547.. [DOI] [PubMed] [Google Scholar]
- 28.Lancerotto L, Chin MS, Freniere B, et al. Mechanisms of action of external volume expansion devices. Plast Reconstr Surg. 2013;132:569–578.. [DOI] [PubMed] [Google Scholar]
- 29.Giatsidis G, Cheng L, Facchin F, et al. Moderate-intensity intermittent external volume expansion optimizes the soft-tissue response in a murine model. Plast Reconstr Surg. 2017;139:882–890.. [DOI] [PubMed] [Google Scholar]
- 30.Lujan-Hernandez J, Lancerotto L, Nabzdyk C, et al. Induction of adipogenesis by external volume expansion. Plast Reconstr Surg. 2016;137:122–131.. [DOI] [PubMed] [Google Scholar]
- 31.Hsiao HY, Liu JW, Brey EM, et al. The effects of negative pressure by external tissue expansion device on epithelial cell proliferation, neo-vascularization and hair growth in a porcine model. PLoS One. 2016;11:e0154328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee JW, Han YS, Kim SR, et al. A rabbit model of fat graft recipient site preconditioning using external negative pressure. Arch Plast Surg. 2015;42:150–158.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Oranges CM, Schaefer DJ. Induction of adipogenesis by external volume expansion. Plast Reconstr Surg. 2016;138:769e–770e.. [DOI] [PubMed] [Google Scholar]
- 34.Chin MS, Ogawa R, Lancerotto L, et al. In vivo acceleration of skin growth using a servo-controlled stretching device. Tissue Eng Part C Methods. 2010;16:397–405.. [DOI] [PubMed] [Google Scholar]
- 35.Greco RJ. Nonsurgical breast enhancement–fact or fiction? Plast Reconstr Surg. 2002;110:337–339.. [DOI] [PubMed] [Google Scholar]
- 36.Smith CJ, Khouri RK, Baker TJ. Initial experience with the Brava nonsurgical system of breast enhancement. Plast Reconstr Surg. 2002;110:1593–1595.; author reply 1595. [DOI] [PubMed] [Google Scholar]
- 37.Khouri RK, Rorich RJ, Baker TJ. Multicenter evaluation of an external tissue expander system (Brava) for breast enlargement. Presented at the 71st Annual Scientific Meeting of ASPS/PSEF/ASMS; November 2–6, 2002; San Antonio, Texas Plast Surg Forum. 2002;96:168–171. [Google Scholar]
- 38.Schlenz I, Kaider A. The Brava external tissue expander: is breast enlargement without surgery a reality? Plast Reconstr Surg. 2007;120:1680–1689.; discussion 1690. [DOI] [PubMed] [Google Scholar]
- 39.Hymes SR, Strom EA, Fife C. Radiation dermatitis: clinical presentation, pathophysiology, and treatment 2006. J Am Acad Dermatol. 2006;54:28–46.. [DOI] [PubMed] [Google Scholar]
- 40.Mojallal A, Lequeux C, Shipkov C, et al. Improvement of skin quality after fat grafting: clinical observation and an animal study. Plast Reconstr Surg. 2009;124:765–774.. [DOI] [PubMed] [Google Scholar]
- 41.Khouri R, Khouri R. Percentage augmentation. Plast Reconstr Surg. 2015;135:933e–935e.. [DOI] [PubMed] [Google Scholar]


