Abstract
Objectives
To describe pancreatic enzyme practices during the first year of life in infants with cystic fibrosis (CF) and evaluate associations between dosing and outcomes, including growth and gastrointestinal symptoms.
Methods
We analyzed data from a subset of infants who were in a prospective cohort study conducted at 28 US CF centers. Anthropometric measurements and medications were recorded at each visit. Diaries with infant diet, pancreatic enzyme replacement therapy (PERT) dosing, stool frequency and consistency and pain were completed by a parent/guardian for three days prior to each visit.
Results
231 infants were enrolled in the main study; 205 of these met criteria for pancreatic insufficiency. PERT dose between birth and 6 months was on average 1882 LU/kg/meal (range: 492 – 3727) and was similar between 6 months and 12 months (mean: 1842 LU/kg/mean, range: 313 – 3612). PERT dose had a weak, negative association with weight z-score at 3 and 6 months (r = −0.16, 95% CI = −0.29, −0.02 and r= −0.18, 95% CI= −0.31, −0.04, respectively) but not at 12 months. There was not a clear relationship between PERT dosing and number of stools/day, stool consistency or pain. 144 infants (70%) were placed on acid suppression medication. Weight z-score mean was 0.37 higher in infants using proton pump inhibitors exclusively vs those using histamine-2 blockers exclusively (95% CI= −0.02, 0.76, p=0.06).
Conclusions
We did not observe that centers with a higher PERT dosing strategy yielded greater clinical benefit than dosing at the lower end of the recommended range.
Keywords: pancreatic enzyme replacement therapy, cystic fibrosis, growth, infancy
INTRODUCTION
Pancreatic enzyme replacement therapy (PERT) is essential for nutrient digestion and absorption required for growth and development in infants with cystic fibrosis (CF) and pancreatic insufficiency (PI), collectively known as CF-PI. Use of PERT has also been critical in preventing early or serious gastrointestinal (GI) complications of malabsorption, diarrhea and vitamin deficiencies in infants diagnosed with CF-PI. Despite the long history of PERT use in infants with CF, the doses used early in life and strategies to change doses are based on consensus recommendations. Those recommendations themselves are based on historical practices..1–3 Most infants in the United States are now diagnosed with CF within the first few weeks of life4,5 and those with PI are treated with PERT at an increasingly younger age3. CF centers across the US implement a wide range of PERT dosing when managing infants with CF to prevent malnutrition, however clear associations between PERT dose and growth in the first year of life are lacking.
The Baby Observational and Nutrition Study (BONUS) was a prospective multi-center study designed to assess growth, nutrition and other clinical outcomes in infants with CF during the first year of life. The primary aim of the study was to define and describe incremental weight gain and linear growth in the first year. As a potential significant modifier of growth, PERT dosing and adjustments based on clinical symptoms and growth parameters were recorded during the study period.
Despite the paucity of data to inform PERT dosing early in life, the underlying pathophysiology suggests that use of PERT is important for optimizing growth, development and overall health in infants with CF-PI. There is clearly a lower limit of efficacy, since lack of endogenous or exogenous (PERT) enzymes leads to severe malnutrition and developmental delays.6–8 Adverse effects have also been documented when exogenous PERT dose is too high with development of fibrosing colonopathy.9 We aimed to use the extensive data collected in BONUS to describe the current state of PERT dosing in CF infants and investigate associations of PERT dosing with growth, feeding type, acid suppression medications and other clinical outcomes. We hypothesized that growth parameters and clinical outcomes are associated with PERT dose.
METHODS
Patients and Study Design
The Baby Observational and Nutrition Study (BONUS) was a prospective cohort study of infants with CF enrolled from birth to 3.5 months of age conducted at 28 US CF centers.10 In brief, exclusion criteria were: <35 weeks gestational age, birth weight < 2.5 kg, or inability to take full oral feeds. Concomitant medications were recorded at each visit, including but not limited to PERT dose and use of acid-suppressing medications. Anthropometric measurements were performed by study certified staff.11 Diaries were completed by a parent/guardian for three days prior to each scheduled clinic visit to collect information regarding infant diet, PERT dosing, meal frequency, stool frequency, stool consistency using the modified pediatric Bristol Stool Form Scale (mpBristol)12 and pain (modified pain diary)13. ESHA Research software was used for nutritional analysis (Salem OR, USA). Participating parent/guardian provided written informed consent and all sites received Institutional Review Board approval (ClinicalTrials.gov NCT01424696).
Definitions and Statistics
World Health Organization (WHO) standard growth curves 14,15 were used to calculate attained weight, length, and occipital-frontal circumference (OFC) for age z-scores. Babies were classified as PI if they had a fecal elastase (FE) result ≤ 200 μg/g or if they had two PI causing CFTR mutations.16 If this information was unavailable, PERT use at study termination was used as a surrogate; all infants who were prescribed PERT are included in these analyses. PERT dose at each visit was calculated as lipase units (LU)/kg/meal, LU/kg/day17. For exclusively formula fed infants, LU/g fat/day was calculated using the average daily fat intake (g) over the 3 day diet diary associated with that visit. PERT dose was summarized using maximum dose by 6 months of age and between 6 and 12 months of age and stratifying into three categories: <1000, 1000–2000, and ≥2000 LU/kg/meal. The frequency and timing of PERT dose adjustments were also calculated per patient. Dose adjustments were categorized as maintenance (± <300 LU/kg/meal), increase or decrease (± ≥300 LU/kg/meal). Rates of dose change were calculated accounting for follow-up time.
As this was an observational study, treating clinicians were free to determine and adjust PERT dose based on their usual clinical practice within their institutions. A post hoc clustered site analysis to compare sites dosing infants with a lower PERT dose (≥85% of patients at site with a median dose<1500 LU/kg/meal) vs. a higher PERT dose (≥85% of patients at site with a median dose ≥1750 LU/kg/meal) was utilized to explore associations between PERT dose and clinical outcomes (weight, length, GI symptoms). Additionally, associations between PERT dosing and GI symptoms, feeding type (breast feeding, formula, or both), CFTR genotype class, meconium ileus, and acid suppressing medications (histamine-2antagonists/blockers [H2 blockers] and proton pump inhibitors [PPI]) were explored.
Summary statistics including means, medians, standard deviations [SD], ranges, and 95% confidence intervals (CI) are reported. Differences and comparisons are made with one and two-sample t-tests, Fisher’s exact test for categorical data, ANOVA, Mann-Whiney test, or Pearson’s correlation coefficient; 95% CIs were calculated and all p-values were two-sided. (SAS V9.4, R V 3.16.)
RESULTS
Cohort Description
Between 2011 and 2014, 231 infants were enrolled in BONUS 10. Of the 231 enrolled BONUS babies, 210 (91.0%) initiated PERT and 205 (97.6%) of those were PI by study definition or were taking PERT; 25 (12.2%) were diagnosed with meconium ileus (MI). Of the 205 babies initiating PERT, 182 (88.8%) had a fecal elastase result ≤200 μg/g, 186 (90.7%) had two class I–III CFTR mutations (126 homozygous F508del), 10 (4.9%) had at least 1 class IV–V mutation, 8 (3.9%) had one class I–III and an unreported/unidentified mutation, and 1 (0.5%) had two unknown/unidentified mutations (Table 1).
Table 1.
Cohort Characteristics by pancreatic status and PERT use among those with PI
| BONUS CF infant Cohort N = 231 |
|||
|---|---|---|---|
|
| |||
| PI + initiated PERT N = 205 |
PI + no PERT use N = 6 |
PS N = 20 |
|
|
| |||
| Fecal Elastase (μg/g) | |||
| ≤50 | 144 (70.2%) | 1 (16.7%) | 0 (0%) |
| 50–≤ 100 | 25 (12.2%) | 0 (0%) | 0 (0%) |
| 100–≤ 200 | 13 (6.3%) | 1 (16.7%) | 0 (0%) |
| >200 | 1 (0.5%) | 2 (33.3%) | 16 (80%) |
| Unavailable | 22 (10.7%) | 2 (33.3%) | 4 (20%) |
|
| |||
| Genotype Class | |||
| I–III/I–III | 186 (90.7%) | 6 (100%) | 3 (15%) |
| IV–V/any | 10 (4.9%) | 0 (0%) | 9 (45%) |
| I–III/unknown | 8 (3.9%) | 0 (0%) | 8 (40%) |
| Unknown/unknown | 1 (0.5%) | 0 (0%) | 0 (0%) |
|
| |||
| Meconium Ileus | |||
| Yes | 25 (12.2%) | 1 (16.7%) | 1 (5%) |
| No | 180 (87.8%) | 5 (83.3%) | 19 (95%) |
|
| |||
| Birth weight z-score (mean, SD) | −0.19 (0.88) | 0.26 (1.17) | 0.08 (1.09) |
|
| |||
| Breastfeeding Status | |||
| Excl BF at 3 mos | 54 (26.3%) | 2 (33.3%) | 1 (5%) |
| BF and FML @ 3 mos | 38 (18.5%) | 0 (0%) | 6 (30%) |
| Excl FML @ 3 mos | 112 (54.6%) | 3 (50.0%) | 12 (60%) |
|
| |||
| Acid Blocker Usage | |||
| None | 61 (29.8%) | 5 (83.3%) | 12 (60%) |
| PPI only | 34 (16.6%) | 0 (0%) | 3 (15%) |
| H2 Blocker only | 63 (30.7%) | 0 (0%) | 5 (25%) |
| PPI + H2 | 47 (22.9%) | 1 (16.7%) | 0 (0%) |
|
| |||
| Participant specific max PERT dose 0–6 mos | |||
| Lipase Units/Kg/Meal | |||
| N | 205 | ||
| mean (sd) | 1882 (621) | ||
| min, max | 492, 3727 | ||
| <1000 | 18 (8.8%) | ||
| 1000–2000 | 99 (48.3%) | ||
| >2000 | 88 (42.9%) | ||
|
| |||
| Participant specific max PERT dose 6–12 mos | |||
| Lipase Units/Kg/Meal | |||
| N | 196 | ||
| mean (sd) | 1842 (617) | ||
| min, max | 313, 3612 | ||
| <1000 | 20 (9.8%) | ||
| 1000–2000 | 90 (43.9%) | ||
| >2000 | 86 (42.0%) | ||
PI=pancreatic insufficient; PS= pancreatic sufficient; SD=standard deviation; Excl= exclusive; BF=breast fed; FML=formula fed; PPI=proton pump inhibitor
PERT Use and Dosing
In this cohort, PERT was always initiated before 5 months of age with a mean age of 2.0 months (range: 0.0 – 4.3). Two PERT brands were predominant (>95%), with remainder shared among two other brands. Ten percent of babies switched brands at least once in the first year of life. Each participants’ maximum PERT dose between birth and 6 months was on average 1882 LU/kg/meal (range: 492 – 3727) and was 12259 LU/kg/day (range: 2358–28139). The dose per meal was similar between 6 months and 12 months (mean: 1842 LU/kg/meal, range: 313 – 3612; the mean daily dose was somewhat lower (10689 LU/kg/day, range: 1660–26388). On average babies ate 6.3 times/day at 3 months (range: 3–12), 5.9 times/day at 6 months (range: 1–12), and 5.5 times/day at 12 months (range: 3–12). The majority of participants were treated with a PERT dose exceeding 2000 LU/kg/meal (55%), while 5% were dosed with less than 1000 LU/kg/meal (Table 1). Maximum PERT dose over the first year of life was similar across genotype classes and breast-feeding status (see online Figure, Supplemental Digital Content 1). Among exclusively formula fed babies, average maximum PERT dose was 2815 (SD=2254) LU/g fat/day (range: 421–12459) in the first 6 months (n=134), and 2941 (SD=1997) LU/g fat/day (range: 384–12422) between 6 and 12 months of age (n=155).
PERT dose and association with Weight and GI Symptoms
PERT dose (LU/kg/meal) had a weak, negative association with weight z-score at 3 and 6 months (r = −0.16, 95% CI = −0.29, −0.02 and r= −0.18, 95% CI= −0.31, −0.04, respectively) but not at 12 months (Figure 1a–c). The average 6-month weight z-scores were slightly lower in those receiving the higher range of PERT dosages during the year (>2000 LU/kg/meal 6 month weight z= −0.64 compared to z= −0.44 for 1000–2000 LU/kg/meal and z= −0.26 for <1000 LU/kg/mean, p=0.26). A similar trend was seen for 12-month weight z-scores (>2000 LU/kg/meal 12 month weight z= −0.17 compared to z= 0.03 for 1000–2000 LU/kg/meal and z= 0.13 for <1000 LU/kg/mean, p=0.27). PERT dosage was not associated with the number of stools reported per day at 6 months (p=.60) or 12 months of age (p=0.75). Stool consistency as reported by the mpBristol (range: 1=”separate hard lumps”, 5=”watery, no solid pieces”) was mean (SD): 4.04 (0.78) 3 months, 3.81 (0.72) at 6 months, and 3.36 (0.96) at 12 months. Overall reported pain was low (mean <1 on scale from 0–10 where 0= no pain), and pain at 12 months was similar among the babies with lower versus higher maximum PERT dose (>2000 LU/kg/meal average pain score= 0.35 compared to 0.51 for 1000–2000 LU/kg/meal and 0.60 for <1000 LU/kg/mean, p=0.46).
Figure 1.
Weight for age z-score by PERT dose at (a) 3, (b) 6, and (c) 12 months.
Of the 478 recorded PERT dose changes during the study interval up to 12 months of age, 58% (n=276) were classified as maintenance (dose change± <300 LU/kg/meal) whereas 34% (n=164) were classified as dose increases and 8% (n=38) as dose decreases. On average, babies had 1.88 maintenance dose changes, 1.4 dose increases and 1.09 dose decreases during the follow-up period. Thirty-six percent (74/205) did not have any dose change; 117 (57%) had at least one dose increase and 35 (17%) had at least one dose decrease while 21 (10%) had both. More frequent dose changes were associated with lower weight (r= −0.17 p=0.02 at 6 months and r= −0.22, p=0.003 at 12 months) and less weight gain between 3 and 12 months (−0.64 weight z-score units per active dose change, 95% CI −1.1, −0.2; p=0.006). There were no clear associations between active dose changes and pain or number of stools.
Use of Acid Suppression
Of the 205 PI infants in the BONUS cohort, 144 (70%) were placed on acid suppression medication at some time during the first year of life; over half (77/144) started acid suppression before enrolling into BONUS (mean age of enrollment 2.6 months10). Sixty-three (30.7%) were exclusively on H2 blockers, 34 (16.6%) received only PPIs, and 47 received both (22.9%). Among those who received both, 83% initiated H2 blockers on average 1 month before PPI. Overall, babies were on H2 blockers for an average of 26 weeks (n=110, SD=15.3), and PPIs for an average of 31 weeks (n=81, SD=14.1). Half of babies only using PPIs had a twelve-month weight-for-age z-score >0, whereas 36.5% of babies using exclusively H2 blockers achieved weight-for-age z-score >0 at 12 months (see Table, Supplemental Digital Content 3) (z-score mean 0.37 higher in PPI vs H2 only, 95% CI= −0.02, 0.76, p=0.06). Babies on both H2 blockers and PPI did not have improved weight at 12 months compared to those on H2 blockers alone (mean z-score difference: −0.03, 95% CI= −0.41, 0.35, p=0.86). Fewer babies on PPI had a maximum dose > 2000 LU/kg/meal at 6 and 12 months than those on H2 blockers only (see Table, Supplemental Digital Content 3).
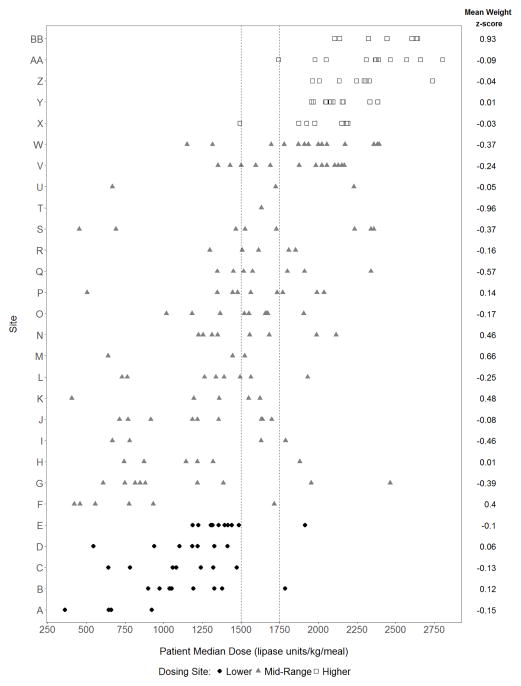
Higher dosing sites versus lower dosing sites
PERT dosing practices varied by site. Five sites (n=43) met the definition of higher PERT dosing (≥85% of participants’ median PERT dose was ≥1750 LU/kg/meal), while 5 sites (n=36) were dosing PERT in a lower range (≥85% of participants’ median PERT dose was <1500 LU/kg/meal) (Figure 2). All others were classified as mid-range. Site characteristics are shown online (see Table, Supplemental Digital Content 4). Gender, genotype distribution, breast feeding, birth weight, incidence of meconium ileus, and preference for PERT brand were similar. Use of proton pump inhibitors alone was more common at lower dosing and mid-range dosing sites, although no acid blocker use, use of H2 blockers alone and the combination of H2 blockers and PPIs was similar across all sites.
Figure 2.
Participant median dose (over entire year) and mean 12 month weight for age z-score by Site. White squares= higher dosing sites’ participants; black circles=lower dosing sites’ participants; gray triangles=mid-range dosing sites’ participants
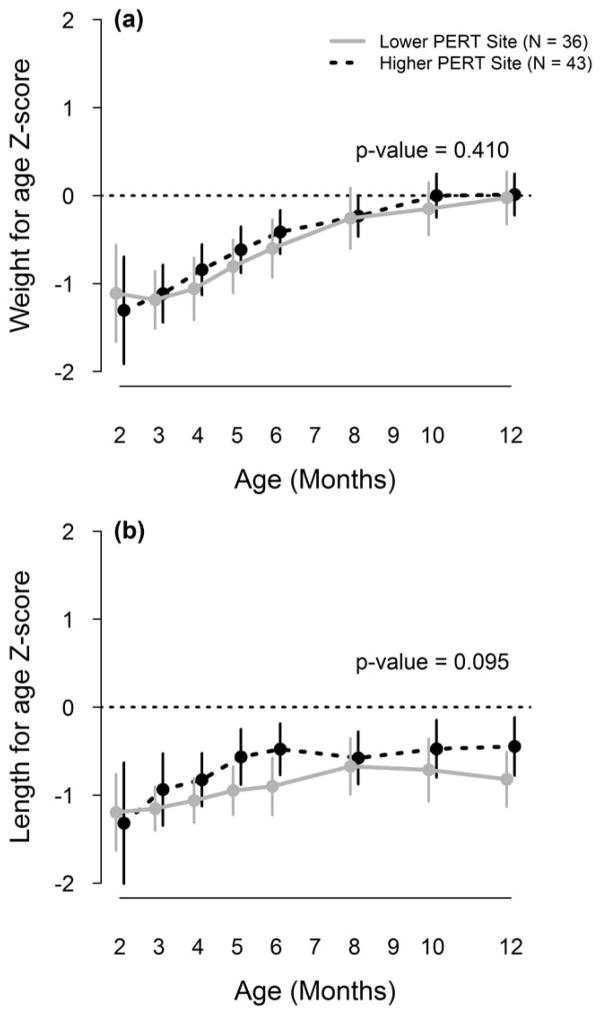
Weight for age z-score did not differ significantly by lower versus higher PERT dosing (p=0.41, Figure 3a) at 12 months where average in the lower dosing sites was −0.03 (95% CI= −0.32, 0.27) compared to 0.01 (95% CI = −0.22, 0.25) in the higher dosing sites. Average weight for age z-score at the mid-range site was −0.11 (95% CI= −0.29, 0.07). There were no overall differences in length for age z-score by lower versus higher dosing strategy (p=0.095, Figure 3b). The 12 month length-for age z-score in the lower dosing sites was −0.82 (95% CI= −1.13, −0.51) compared to −0.45 (95%CI= −0.78, −0.12) in the higher dosing sites, and −0.65 (95% CI= −0.83, −0.47) in the mid-range dosing sites.
Figure 3.
Average weight and length for age over the first year of life by site PERT dosing. Gray solid line= lower PERT dosing sites; black hashed line= higher PERT dosing sites; p-value is for overall test of difference between curves.
There was not a clear relationship between PERT dosing and GI symptoms. The distribution of stool consistency (mpBristol) did not differ across lower dosing and higher dosing sites at either 6 or 12 months (p = 0.33, p = 0.70). At 3 months, there was a trend toward watery stools (mpBristol type = 5) in the babies at lower dosing sites (33.3%) vs higher (11.6%; p = 0.03). There was more laxative use at the lower dosing sites. Serum alpha-tocopherol levels were higher in the higher dosing sites at 6 months but were within the normal range at lower dosing sites; that difference was attenuated at 12 months (see Table, Supplemental Digital Content 4).
DISCUSSION
The data from this prospective, observational study indicate that clinicians prescribe a wide range of PERT doses to infants in the first year of life. Mean dose per meal was in the higher mid-range. The slightly higher total daily dose in the first six months of life likely is consistent with the increased number of feedings during this early time of life17. Despite longstanding practice and consensus recommendations to “optimize” PERT dosing18, we did not observe a dose-response effect on weight gain or height in this large cohort of infants assessed at 3, 6 and 12 months of life. Although the average 6-month weight z-scores were lowest in those receiving higher PERT dosages, this likely represents indication bias rather than cause and effect as practitioners may perceive that weight is faltering due to reduced fat absorption, leading them to increase PERT dose. One might also consider a similar clinical bias at the lower end of the dosing range as well – that is, these infants are growing well and thus the dose was not increased. Using differences in practice patterns by site, we were able to conduct a natural experiment comparing PERT dosing strategies on weight gain and linear growth. In this setting, where indication bias is presumably diminished, weight and length for age z-score did not differ significantly between sites that dosed PERT at the lower end of the recommended range versus those who tended to dose at the higher end, therefore strengthening our observation of lack of effect of PERT dose on growth in BONUS infants.
CF clinicians have been trained to increase PERT dose in response to frequent or loose stools and have worried that higher doses of PERT can cause constipation or lead to fibrosing colonopathy. Based on parent/guardian diaries, PERT dosage and change was not associated with number of stools per day. Although there appears to be some trends toward softer stool consistency (mpBristol) at the lower dosing sites at 3 months, the differences go away by 6 and 12 months. Of note, lower dosing sites tended to use more laxatives. This study did not collect information on the rationale for use of laxatives; no correlation was seen between stool consistency and laxative use. There are no specific recommendations about the use of laxatives in infants with CF3. The amount of pain reported and the odds of more intense pain tended to be less among the babies with higher maximum PERT dose; however, our pain scale was not specific to abdominal pain. As with the number of stools, there was no clear association between active dose changes and pain scores.
Delayed buffering of gastric pH has been observed in adults with CF19 and a higher luminal pH (>5.5) is needed for optimal dissolution of enteric coating of PERT1,20. In addition, gastro-esophageal reflux may lead to feeding difficulties. Both of these theoretical rationales likely contributed to the 70% use of any acid blockade in these infants with CF who are at risk for poor growth. This rate of acid blockade use is more than twice as high as that observed in a cohort of premature infants, although in that study these medicines were likely used exclusively for treatment of GER21. We have previously shown that use of H2 blockers, either alone or in combination with a PPI, are associated with increased risk of lower weight or length in this cohort 10. In this further analysis, we observed that at one year of age, half of babies using PPIs exclusively had a weight-for-age z-score above the mean for the healthy population versus 36% of those on H2 blocker alone. PPIs act by irreversibly blocking the hydrogen-potassium adenosine triphosphatase enzyme system (H+/K+ ATPase) of gastric parietal cells as well as other cells that generate hydrogen potassium gradient. Complications in bone health as well as other immune mediated dysregulation has been linked to the changes induced by PPI22. H2 receptors are present in parietal cells and when blocked, gastric acid secretion is minimized. However, H2 receptors are also present in other tissues and hematopoetic cells. H2 blockers may suppress IL-12 and stimulate IL-10 production23. Decreased production of IL-10 in CF bronchi has been suggested as a mechanism that contributes to increased local inflammation and tissue damage24. One might speculate that non-acid-blocking effects of H2 blockers could be a factor in suboptimal growth. Nonetheless, our observations indicate that more guidance is needed if acid suppression therapy is used in infants with CF.
An observational study cannot prove cause and effect; however we have not identified any clear associations between PERT dose with infant weight gain and GI symptoms. By coincidence, sites self-segregated based on their PERT dosing, giving us a post-hoc opportunity to examine and confirm the relationship between higher and lower PERT and outcomes without the confounding effect of indication bias. This designed allowed us to look for a PERT dose effect and did not observe one; however almost all CF infants were dosed within the recommended range, therefore there may be threshold for PERT efficacy at the lower end of that range. A dose escalation study would determine whether a dose plateau exists with PERT use. Use of H2 blockers or PPI for acid blockade may have been influenced by clinician practice or by payor preference. The use of both H2 blockers and PPI in combination may indicate a suboptimal “step up” strategy; our observations suggest that there is no clinical benefit to this approach.
Although our findings are contrary to standard PERT recommendations in infants, they represent the best evidence to date concerning PERT dosing and clinically relevant endpoints. Improved growth early in life is associated with better clinical outcomes later in life25, thus if an optimal infant dose of PERT could be determined it would be an important therapeutic recommendation. A recent review of older individuals in the US Cystic Fibrosis Patient Registry indicated that a somewhat higher mid-range dose of PERT was associated with better growth and BMI than a lower mid-range dose.26 The mean dose of PERT used in this cohort was in the higher mid-range and the majority of infants with PI enrolled in a BONUS study grew well10; however we did not observe that a higher PERT dosing strategy yielded greater benefit than dosing at the lower end of the recommended range.
Supplementary Material
What is known
Cystic fibrosis (CF) causes pancreatic insufficiency and complications early in life.
Use of pancreatic enzyme replacement therapy (PERT) is common in CF but optimal dosing and how it relates to infant growth is unknown.
Use of acid suppression is common in infants with CF although clinical correlates have not been well-defined.
What is new
Infants with CF who are pancreatic insufficient receive a wide range of PERT dosing across US institutions.
In this observational study, there is no association between PERT dosing and growth parameters.
Use of acid suppressing medication was common in this cohort and there was an association with better weight z-score at 12 months with proton pump inhibitor (PPI) use compared to histamine-2 blockers.
Acknowledgments
We thank the CF Foundation Therapeutic Development Network sites, participating patients and families, and the CF Foundation Patient Registry for their contributions to this study.
Funding/support: BONUS and its lead investigators were supported by CFFT BONUS11KO, NIH R01DK095738, NIH P30DK089507, NIH UL1TR000423, CFF LEUNG14GE0 and NIH P30 DK072482.
Footnotes
Author Contributions: Design and Conduct of the Study: Gelfond, Heltshe, Borowitz, Leung, Heubi, Ramsey; Collection, management, analysis and interpretation of data: All authors; Preparation, review or approval of the manuscript: All authors; Statistical Analysis: Heltshe, Skalland, Kloster
Conflict of interest disclosures and Sources of Funding: Dr Gelfond has served as a consultant for Vertex, Abbvie and Chiesi Pharmaceuticals. Dr. Leung has served as a consultant for Vertex and receives research/grant support from Bristol Meyers Squibb, Gilead, Abbvie, and Roche pharmaceuticals outside of the submitted work. Dr. Ramsey discloses that over the past 3 years she has received grant support from the Cystic Fibrosis Foundation and the National Institutes of Health. She has been the principal investigator on contracts between Seattle Children’s Hospital and the following companies: Aridis Pharmaceuticals, LLC, Celtaxsys, Kalobios, Flatley Discovery Labs, LLV, Vertex Pharmaceuticals Inc, Laurent Therapeutics, Inc, Nilvalis Therapeutics, Inc, and Synedgen, Inc. Dr Heubi has received personal fees from Alynlam Pharma and has financial interest in Asklepion Pharma LLC. No other disclosures are reported.
References
- 1.Borowitz DS, Grand RJ, Durie PR. Use of pancreatic enzyme supplements for patients with cystic fibrosis in the context of fibrosing colonopathy. Consensus Committee J Pediatr. 1995;127:681–684. doi: 10.1016/s0022-3476(95)70153-2. [DOI] [PubMed] [Google Scholar]
- 2.Stallings VA, Stark LJ, Robinson KA, et al. Evidence-based practice recommendations for nutrition-related management of children and adults with cystic fibrosis and pancreatic insufficiency: results of a systematic review. J Am Diet Assoc. 2008;108:832–839. doi: 10.1016/j.jada.2008.02.020. [DOI] [PubMed] [Google Scholar]
- 3.Borowitz D, Robinson KA, Rosenfeld M, et al. Cystic Fibrosis Foundation evidence-based guidelines for management of infants with cystic fibrosis. J Pediatr. 2009;155:S73–93. doi: 10.1016/j.jpeds.2009.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wagener JS, Zemanick ET, Sontag MK. Newborn screening for cystic fibrosis. Curr Opin Pediatr. 2012;24:329–335. doi: 10.1097/MOP.0b013e328353489a. [DOI] [PubMed] [Google Scholar]
- 5.Rosenfeld M, Sontag MK, Ren CL. Cystic Fibrosis Diagnosis and Newborn Screening. Pediatric Clinics of North America. 2016;63:599. doi: 10.1016/j.pcl.2016.04.004. [DOI] [PubMed] [Google Scholar]
- 6.Andersen D. Cystic fibrosis of the pancreas and its relation to celiac disease: a clinical and pathological study. Am J Dis Child. 1938;56:344–399. [Google Scholar]
- 7.Shwachman H. Progress in the study of “mucoviscidosis” (pancreatic fibrosis); with illustrative case presentations. Pediatrics. 1951;7:153–163. [PubMed] [Google Scholar]
- 8.Kalnins D, Wilschanski M. Maintenance of nutritional status in patients with cystic fibrosis: new and emerging therapies. Drug Des Devel Ther. 2012;6:151–161. doi: 10.2147/DDDT.S9258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.FitzSimmons SC, Burkhart GA, Borowitz D, et al. High-dose pancreatic-enzyme supplements and fibrosing colonopathy in children with cystic fibrosis. N Engl J Med. 1997;336:1283–1289. doi: 10.1056/NEJM199705013361803. [DOI] [PubMed] [Google Scholar]
- 10.Leung DH, Heltshe SL, Borowitz D, et al. Effects of Diagnosis by Newborn Screening for Cystic Fibrosis on Weight and Length in the First Year of Life. JAMA Pediatr. 2017;171:546–554. doi: 10.1001/jamapediatrics.2017.0206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Coburn-Miller C, Casey S, Luong Q, et al. Standardization of Research-Quality Anthropometric Measurement of Infants and Implementation in a Multicenter Study. Clin Transl Sci. 2015;8:330–333. doi: 10.1111/cts.12283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chumpitazi BP, Lane MM, Czyzewski DI, et al. Creation and initial evaluation of a Stool Form Scale for children. J Pediatr. 2010;157:594–597. doi: 10.1016/j.jpeds.2010.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Taddio A, Nulman I, Koren BS, et al. A revised measure of acute pain in infants. J Pain Symptom Manage. 1995;10:456–463. doi: 10.1016/0885-3924(95)00058-7. [DOI] [PubMed] [Google Scholar]
- 14.WHO Mulicenter Growth Reference Study Group. WHO Child Growth Standards: length/height-for-age, weight-for-age, weight-for-height and body mass index-for-age: methods and development. Geneva: World Health Organization; 2006. [Google Scholar]
- 15.Grummer-Strawn LM, Reinold C, Krebs NF. Use of World Health Organization and CDC growth charts for children aged 0–59 months in the United States. MMWR Recomm Rep. 2010;59:1–15. [PubMed] [Google Scholar]
- 16.O’Sullivan BP, Baker D, Leung KG, et al. Evolution of pancreatic function during the first year in infants with cystic fibrosis. J Pediatr. 2013;162:808–812. e801. doi: 10.1016/j.jpeds.2012.10.008. [DOI] [PubMed] [Google Scholar]
- 17.Borowitz D, Gelfond D, Maguiness K, et al. Maximal daily dose of pancreatic enzyme replacement therapy in infants with cystic fibrosis: a reconsideration. J Cyst Fibros. 2013;12:784–785. doi: 10.1016/j.jcf.2013.05.011. [DOI] [PubMed] [Google Scholar]
- 18.Cystic Fibrosis F, Borowitz D, Robinson KA, et al. Cystic Fibrosis Foundation evidence-based guidelines for management of infants with cystic fibrosis. J Pediatr. 2009;155:S73–93. doi: 10.1016/j.jpeds.2009.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gelfond D, Ma C, Semler J, et al. Intestinal pH and gastrointestinal transit profiles in cystic fibrosis patients measured by wireless motility capsule. Dig Dis Sci. 2013;58:2275–2281. doi: 10.1007/s10620-012-2209-1. [DOI] [PubMed] [Google Scholar]
- 20.Kuhn RJ, Eyting S, Henniges F, et al. In Vitro Comparison of Physical Parameters, Enzyme Activity, Acid Resistance, and pH Dissolution Characteristics of Enteric-Coated Pancreatic Enzyme Preparations: Implications for Clinical Variability and Pharmacy Substitution. J Pediatr Pharmacol Ther. 2007;12:115–128. doi: 10.5863/1551-6776-12.2.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.D’Agostino JA, Passarella M, Martin AE, et al. Use of Gastroesophageal Reflux Medications in Premature Infants After NICU Discharge. Pediatrics. 2016:138. doi: 10.1542/peds.2016-1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wu D, Qiu T, Zhang Q, et al. Systematic toxicity mechanism analysis of proton pump inhibitors: an in silico study. Chem Res Toxicol. 2015;28:419–430. doi: 10.1021/tx5003782. [DOI] [PubMed] [Google Scholar]
- 23.Elenkov IJ, Webster E, Papanicolaou DA, et al. Histamine potently suppresses human IL-12 and stimulates IL-10 production via H2 receptors. J Immunol. 1998;161:2586–2593. [PubMed] [Google Scholar]
- 24.Bonfield TL, Konstan MW, Burfeind P, et al. Normal bronchial epithelial cells constitutively produce the anti-inflammatory cytokine interleukin-10, which is downregulated in cystic fibrosis. Am J Respir Cell Mol Biol. 1995;13:257–261. doi: 10.1165/ajrcmb.13.3.7544594. [DOI] [PubMed] [Google Scholar]
- 25.Yen EH, Quinton H, Borowitz D. Better nutritional status in early childhood is associated with improved clinical outcomes and survival in patients with cystic fibrosis. J Pediatr. 2013;162:530–535. e531. doi: 10.1016/j.jpeds.2012.08.040. [DOI] [PubMed] [Google Scholar]
- 26.Haupt ME, Kwasny MJ, Schechter MS, et al. Pancreatic enzyme replacement therapy dosing and nutritional outcomes in children with cystic fibrosis. J Pediatr. 2014;164:1110–1115. e1111. doi: 10.1016/j.jpeds.2014.01.022. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.