Abstract
Background
Pregnant women may be co-exposed to multiple insecticides in regions where both pyrethroids and dichlorodiphenyltrichloroethane (DDT) are used for indoor residual spraying (IRS) for malaria control. Despite the potential for adverse effects on offspring, there are few studies in areas where IRS is currently used and little is known about the effects of pyrethroids on children’s health.
Methods
We investigated the relationship between concentrations of four urinary pyrethroid metabolites in urine and organochlorine pesticide concentrations in maternal blood collected near delivery on body weight and body composition among children ≤2 years old participating in the prospective South Africa VHEMBE birth cohort (N=708). We used measurements of length/height and weight collected at 1 and 2 years of age to calculate body mass index (BMI)-for-age, weight-for-age, and weight-for-height z-scores based on World Health Organization standards. We fit separate single-pollutant mixed effects models for each exposure of interest and also stratified by sex. We also fit all analyte concentrations jointly by using a Bayesian kernel machine regression (BKMR) statistical method to assess variable importance of each analyte and to explore the potential for joint effects of the multiple exposures.
Results
Single-pollutant linear mixed effects models showed that, among girls only, p,p′-DDT was associated with higher BMI-for-age (adjusted [a]β=0.22 [95%CI: 0.10, 0.35]; sex interaction p-value=0.001), weight-for-height (aβ=0.22 [95%CI: 0.09, 0.34]; sex interaction p-value=0.002), and weight-for-age (aβ=0.17 [95%CI: 0.05, 0.29], sex interaction p-value=0.01). Although single-pollutant models suggested that p,p′-DDT and dichlorodiphenyldichloroethylene (p,p′-DDE) was also associated with these outcomes in girls, p,p′-DDE was no longer associated in multi-pollutant models with BKMR. The pyrethroid metabolites cis-(2,2-dibromovinyl)-2,2-dimethylcyclopropane-1-carboxylicacid (cis-DBCA) and trans-3-(2,2-dichlorovinyl)-2,2-dimethylcyclopropane carboxylic acid (trans-DCCA) were inversely related to BMI-for-age and weight-for-height overall; however, results suggested that weight-for-age and weight-for-height associations for trans-DCCA (sex interaction p-valueweight-for-age=0.02; p-valueweight-for-height=0.13) and cis-DCCA (sex interaction p-valueweight-for-age=0.02; p-valueweight-for-height=0.08) were strongest and most consistent in boys relative to girls. BKMR also revealed joint effects from the chemical mixture. For instance, with increased concentrations of p,p′-DDE, the negative exposure-response relationship for cis-DBCA on BMI-for-age became steeper.
Conclusions
Our single-pollutant and multi-pollutant model results show that maternal serum p,p′-DDT concentration was consistently and positively associated with body composition and body weight in young girls and that maternal urinary pyrethroid metabolite concentrations (particularly cis-DBCA and trans-DCCA) were negatively associated with body weight and body composition in young boys. Joint effects of the insecticide exposure mixture were also apparent, underscoring the importance of using advanced statistical methods to examine the health effects of chemical mixtures.
Keywords: DDT, DDE, indoor residual spraying, prenatal, pyrethroids
1. Introduction
Low- and middle-income countries in sub-Saharan Africa are undergoing rapid development and urbanization. This rapid transition is bringing with it a ‘double burden’ of co-existing malnutrition (defined by stunting/underweight) and overweight/obesity epidemics, which according to the World Health Organization (WHO), represent a growing public health threat to the African sub-continent (WHO, 2016a). Children under 5 years of age from the southern Africa sub-region have a higher prevalence of overweight (weight-for-height + 2 standard deviations (SD) of WHO growth standards (WHO, 2012)) than all other sub-regions in the world in this age group (WHO, 2016b), with prevalence being particularly high among girls (Pienaar, 2015; Sartorius et al., 2015). The prevalence of underweight (weight-for-height - 2SD of WHO growth standards (WHO, 2012)), at 10% (WHO, 2016b), is high as well and is higher in South African boys compared to girls (Kimani-Murage et al., 2010; Kruger, 2014).
Early-life exposure to endocrine disrupting chemicals (EDCs) has been hypothesized to play a role in the increasing rates of overweight and obesity globally (Braun, 2016; Chevalier and Fénichel, 2016). Indoor residual spraying (IRS), which involves the application of insecticides, such as dichlorodiphenyltrichloroethane (p,p′-DDT) or pyrethroids, on walls, ceilings and eaves of residences, is currently used for mosquito control in most malaria-endemic areas, including South Africa (Maharaj et al., 2016; van den Berg, 2009). In vitro studies show that both p,p′-DDT and its breakdown product, dichlorodiphenyldichloroethylene (p,p′-DDE), promote adipocyte cell growth, differentiation and/or dysfunction (Howell and Mangum, 2011; Kim et al., 2016; Moreno-Aliaga and Matsumura, 2002). p,p′-DDT is a strong agonist of estrogen (Shekhar et al., 1997), which is known to be involved in deposition, differentiation, and metabolism of adipose tissue (Pallottini et al., 2008), and p,p′-DDE demonstrates both anti-androgen and estrogenic activity (Kelce et al., 1995; Sohoni, 1998). Emerging evidence from in vitro and in vivo studies also show that IRS pyrethroids and their metabolites may be EDCs (Brander et al., 2016), suggesting that pyrethroids may also play a role in impacting child body composition.
Several epidemiologic studies have shown that prenatal measures of exposure to DDE is associated with higher body weight and body mass index (BMI) in children, and that these positive associations may be sex-specific (Agay-Shay et al., 2015; Delvaux et al., 2014; Heggeseth et al., 2015; Iszatt et al., 2015; La Merrill and Birnbaum, 2011; Lee et al., 2011; Tang-Peronard et al., 2014; Vafeiadi et al., 2015; Valvi et al., 2011, 2015; Verhulst et al., 2009; Warner et al., 2014). However, other studies did not observe an association (Cupul-Uicab et al., 2010, 2013; Garced et al., 2012; Gladen et al., 2004; Høyer et al., 2014; Karlsen et al., 2016; Tang-Peronard et al., 2014; Warner et al., 2013) or have observed a sex-specific inverse association (de Cock et al., 2014). Most studies have occurred in middle- or high-income countries usually when DDT was no longer in use. In addition, no published epidemiologic studies have investigated associations between prenatal pyrethroid exposure and body weight or body composition in childhood nor investigated associations between joint prenatal exposure to DDT/E and pyrethroids, which is especially relevant in the IRS context where co-exposures to these insecticides are likely to occur (Bouwman et al., 2006).
In the present study, we investigated associations between biomarker concentrations of DDT and DDE and pyrethroid metabolites in mothers near delivery in relation to body weight and body composition in their children at 1- and 2-years, in a longitudinal birth cohort in Limpopo, South Africa. We also explored the joint effects of exposure to both classes of insecticides using a multipollutant Bayesian statistical method (Bobb et al., 2015; Coker et al., 2017; Valeri et al., 2017). We have previously shown in this cohort that IRS-treated homes have higher dust contamination of DDT and DDE which are related to higher maternal serum levels (Gaspar et al., 2015).
2. Methods
2.1. Study Participants and data collection
Data came from the Venda Health Examination of Mothers, Babies and their Environment (VHEMBE) study, a longitudinal birth cohort of mother-child pairs living in the Vhembe district of South Africa’s Limpopo Province. We recruited pregnant women presenting with signs of labor at Tshilizidini hospital, in the city of Thohoyandou between 2012 and 2013. Women were eligible if they were ≥18 years of age, lived in a home where the primary spoken language was Tshivenda, lived <20 km from the hospital, had no intention of moving away from the area during the following 2 years, had no malaria diagnosis during pregnancy, had contractions more than five minutes apart, and delivered a live singleton infant. Of the 1649 women approached for participation in the study, 920 were eligible and 752 were enrolled. Of the children enrolled, 3.2% (n=24) died by the 2nd year follow up (4 died within the first week of life, 13 died between delivery and 1 year, and 7 died between 1 and 2 years). Finally, a total of 708 children had anthropometric measures taken during at least one or both follow-up visits conducted around ages 1- and 2-years. The sample that did not complete anthropometric measurements at both visits (N=44) were similar to the final study population on socio-demographic factors. Maternal written consent was obtained before participant enrollment and human subject research was approved by the Institutional Review Boards at the University of California, Berkeley, McGill University, the University of Pretoria, the Limpopo Department of Health and Social Development, and the Ethics Committee of Tshilidzini Hospital.
Shortly after delivery and at each follow-up visit, the mother or primary caretaker was interviewed using a structured questionnaire administered in Tshivenda (the local language) by bilingual interviewers. We collected information on household and maternal socio-demographic characteristics, and maternal and child health. Maternal and pediatric medical records were also abstracted for important indicators of maternal and child health (e.g., prescription of anti-retroviral therapy for HIV). At the initial interview, the mother’s height and weight were measured, and the mother’s weight was obtained at each subsequent visit. Birthweight was measured in the hospital using a digital neonatal scale (Tanita BD-815U scale, Arlington Heights, Il, USA) and weight measures were made at the 1- and 2-year visits using a pediatric digital scale (Tanita BD-590 Pediatric Scale). Infant length was measured in the hospital soon after birth and at the 1-year visit using a portable infantometer (Seca 417 Measuring Board, Chino, CA, USA) and height at the 2-year visit using a stadiometer (Seca 213 Measuring Board). Triplicate height measures were averaged for each time point. Weight-for-age z-scores, weight-for-height z-scores, and BMI-for-age z-scores were calculated for 1- and 2-year visits using the WHO reference weight growth curves by age (in days) and sex (WHO, 2011). BMI was calculated by dividing each child’s weight measurement by the square of the length or height measurement (kg/m2).
2.2. Exposure Assessment of DDT/E and Pyrethroids
Blood samples were collected from mothers by study nurses either just before (N=595) or soon after delivery (N=157). Each blood sample was divided into clot and serum and stored at −80°C in the study’s field office freezer on hospital grounds. Serum aliquots of 2 mL each were placed on dry ice and shipped to Emory University (Atlanta, GA) for analysis of p,p′-DDT/E and o,p′- DDT/E by high resolution gas chromatography-isotope dilution mass spectrometry (GC-MS) (Barr et al., 2003). DDT and DDE concentrations were expressed on a lipid basis (ng/g lipid) for statistical analysis purposes. DDT/E analytes required a lipid-adjustment to account for differences in partitioning in tissue of these analytes based on variation in lipid levels (Meeker et al., 2007). Triglycerides and cholesterol concentrations were measured in serum samples using standard enzymatic techniques (Roche Chemicals, Indianapolis, IN), and total lipids were estimated using the formula of Phillips et al. (Phillips et al., 1989). The o,p′-DDT and o,p′-DDE isomers were not included in our statistical analyses because >50% of the samples were below the LOQ (see Table 2 for limits of detection [LOD] of DDT/E analytes). Quality-control (QC) procedures included field spikes, field and trip blanks, matrix-matched calibrants, and laboratory-prepared serum and reagent blanks analyzed along with study samples. Further details of the sampling and analysis protocol and QC results are presented in Gaspar et al., (2017). All blank measurement values were below detection limits.
Table 2.
Summary statistics of prenatal insecticides exposures measured in maternal blood (organochlorines, unadjusted/adjusted for ng/g lipids) or urine (pyrethroid metabolites, unadjusted/adjusted for specific gravity) among children who completed the 1st and/or 2nd-year visits between 2012 and 2015, Limpopo, South Africa.
| Insecticides | n (%) above LODa | Unadjusted
|
Adjusted
|
|---|---|---|---|
| Geometric Mean (±GSD)b | Geometric Mean (±GSD)c | ||
| Organochlorines
| |||
| p,p′-DDT | 695 (98.2) | 477.8 (6.64) | 68.94 (6.65) |
| p,p′-DDE | 708 (100) | 1978.90 (4.81) | 285.52 (4.82) |
|
Pyrethroid metabolites | |||
| cis-DBCA | 698 (100) | 0.23 (3.43) | 0.22 (3.42) |
| cis-DCCA | 698 (100) | 0.31 (2.96) | 0.31 (2.95) |
| trans-DCCA | 698 (100) | 0.36 (3.44) | 0.36 (3.43) |
| 3-PBA | 697 (99.7) | 0.72 (2.81) | 0.71 (2.80) |
Limit of Detection (LOD) for p,p′-DDT=0.01ng/mL; LOD for p,p′-DDE= 0.03ng/mL; LOD for cis-DBCA =0.0025 μg/L ; LOD for cis-DCCA=0.0045 μg/L; LOD for trans-DCCA=0.0038 μg/L; LOD for 3-PBA= 0.0047 μg/L
Organochlorine units: pg/g serum; Pyrethroid units: μg/L
Organochlorine units: ng/g lipids; Pyrethroid units: μg/L
GSD = Geometric Standard Deviation
Maternal spot urine samples were collected at the hospital just before (N=434) or after (N=264) delivery (n=10, did not have urine collected). Specific gravity was measured at the time of urine collection using an Atago PAL-10S refractometer (Tokyo, Japan). Urine samples were subsequently stored at −80°C until shipment on dry ice to the Centre de Toxicology du Québec (Québec, Canada) for analysis of five different pyrethroid metabolites by gas chromatography-mass spectrometry (GC-MS) (Dewailly et al., 2014). We measured the following pyrethroid metabolites; cis-(2,2-dibromovinyl)-2,2-dimethylcyclopropane-1-carboxylicacid (cis-DBCA), cis-3-(2,2-dichlorovinyl)-2,2-dimethylcyclopropane carboxylic acid (cis-DCCA), trans-3-(2,2-dichlorovinyl)-2,2-dimethylcyclopropane carboxylic acid (trans-DCCA), 3-phenoxybenzoic acid (3PBA), and 4-fluoro-3-phenoxybenzoic acid (4F3PBA) (see LODs in Table 2). The 4F-3-PBA metabolite was not included in statistical analyses because >50% of urine concentrations were below the LOQ. Metabolite concentrations were specific gravity-standardized to account for variation in urinary dilution. Internal quality controls were implemented using homemade reference materials and ClinCheck non-certified reference materials in urine (RECIPE; Munich, Germany). The laboratory conducting pyrethroid analyses twice-annually participates in the German External Quality Assessment Scheme (G-EQUAS; Erlangen, Germany) to assure overall quality and accuracy. Field and trip blanks were also collected and targeted pyrethroid metabolites were all below detection limits. The recoveries from spiked samples for each analyte ranged between 76 to 86 %. The intra-day precision (repeatability) of the method was between 2.2 to 5.0 % and the inter-day precision (reproducibility) was between 3.0 to 7.7 % depending on the analyte.
2.3. Description of covariates
We used directed acyclic graphs (DAG) to select potential confounders for the regression models (see Supplemental Figure S1). The following household and maternal covariates were identified: household food poverty status at delivery (below food poverty versus above poverty); maternal parity (primiparous versus multiparous); maternal education (<12th grade; 12th grade completed; further studies started; diploma or further degree); postpartum maternal BMI (continuous); maternal HIV status; and maternal age (years-continuous). Maternal HIV status was determined from maternal interviews and a review of maternal medical records for prescriptions of anti-retroviral medications. Household food poverty status was ascertained from reported household income and number of people supported and was compared to South Africa’s national food poverty line thresholds (e.g., 386 rands/month/person; (Werner Ruch, Manager Statistics SA, personal communications, 2016)).
We also tested for effect modification of associations on biologically relevant factors. Specifically, we tested whether child sex, maternal HIV status, caloric/energy intake during pregnancy, household food insecurity during pregnancy, or household poverty status modified associations between insecticides and body weight or body composition. We also tested whether insecticides from different chemical classes exhibited interaction effects (described in detail in Statistical Analysis section). Maternal energy intake was measured in kilojoules (kJ) and determined by a nutritionist based on maternal answers on a food frequency questionnaire (South Africa Medical Research Council/WAMTechnology cc; Stellenbosch, South Africa). A mother was considered malnourished if her daily total caloric intake was below the Institute of Medicine’s dietary reference values for late-term pregnancy (<11,000 kJ) (Otten et al., 2006). We determined food insecurity during pregnancy from maternal interviews, using the US Department of Agriculture’s six-item short form to construct a household food security index. Food security index values ≥2 were considered to be food insecure (range of 0–6).
2.5. Statistical Analyses
Summary statistics were computed for all study outcomes, exposures, and covariates of interest. We calculated Pearson’s correlations between serum log-10-transformed concentrations of p,p′-DDT and p,p′-DDE lipid-adjusted and pyrethroid specific gravity standardized metabolites. We also used the log-10 transformed prenatal insecticide concentrations for regression analyses to reduce the influence of outliers. Serum samples without detectable levels of p,p′-DDT (n=13) were imputed by randomly selecting from below the limit of detection (LOD, see Table 2) on a log normal distribution whose parameters were determined by maximum likelihood estimation (Lubin et al., 2004). With the lmer function in R, we applied linear mixed effects regression modeling to estimate the crude and adjusted associations between prenatal insecticide exposure and child body weight and body composition measures. These models were run for the entire study population and stratified by sex. We accounted for correlation of repeated body weight and body composition measures within participants using a random intercept for each child and controlling for baseline confounders. Hence we interpret the exposure-response coefficient as the difference in mean body weight or body composition measure (e.g., weight-for-age z-score) for a 10-fold increase in prenatal exposure (e.g. p,p′-DDT). Main effects from conventional linear mixed effects regression were considered statistically significant at a p-value<0.05 while interaction effects were considered statistically significant at a p-value<0.1. For each insecticide exposure measure we also tested the linearity assumption by fitting generalized additive models (GAMs) (thin plate regression spline basis) and compared the GAM models with linear models at age 1 and 2 years. Smoothed regression spline terms were considered to potentially violate the non-linearity assumption at a p-value<0.1 using ANOVA for model comparisons.
We also used Bayesian Kernel Machine Regression (BKMR), a non-parametric Bayesian variable selection framework that allowed us to explore joint (or combined) effects of the multiple chemicals. Compared against linear regression methods (Agier et al., 2016; Bobb et al., 2015; Kwon et al., 2011), a Bayesian framework for identifying the optimal set of exposure predictors in a high-dimensional setting has been shown to perform well, while effectively accounting for model uncertainty by way of Bayesian model averaging (see Supplemental Materials for a fuller description on BKMR) (Agier et al., 2016; Kwon et al., 2011; Yi et al., 2005). Briefly, BKMR combines Bayesian and statistical learning methods to iteratively regress a response variable on a nonparametric term of exposure mixture components, specified by a Gaussian kernel function. The model for non-linearity in the exposure-specific responses and thus avoids some of the modeling assumptions of parametric regression methods (Bobb et al., 2013). Additionally, the credible intervals computed in BKMR build in additional uncertainty in the model-fitting process in order to account for the high dimensionality of multiple exposures, thus applying a penalty due to multiple testing (Scott and Berger, 2010; Valeri et al., 2017). BKMR also addresses collinearity of exposures using a hierarchical variable selection procedure (Bobb et al., 2015). With hierarchical variable selection, group and conditional indicator variables are averaged across the MCMC iterations to derive the posterior means that represent group-posterior inclusion probabilities (PIPs) and conditional (within-group)-PIPs (Bobb et al., 2015; Coull et al., 2015; O’Hara and Sillanpää, 2009).
We stratified BKMR analyses by sex and controlled for the same covariates and subject random intercept as in the linear mixed effects models. We applied hierarchical variable selection, grouping DDT/E together and the pyrethroids together, allowing us to identify the most important class of insecticides (DDT/E versus pyrethroids) within the mixture and the most important correlated exposures within a class of insecticides. Models were run at 25,000 iterations, with default values for the ‘slab-and-spike’ priors. We present the group-PIPs and conditional-PIPs from BKMR to describe the relative importance of each prenatal insecticide exposure for each study outcome, using a PIP threshold of 0.5 (Berger and Barbieri, 2004) to draw inference on variable “importance”. We also present graphical output generated by BKMR exposure response surfaces to highlight the exposure-response relationships for each exposure component in the prenatal insecticide mixture when holding all other exposures of interest at the median or at varying levels of combined exposure levels (range= 5th to 95th percentile of combined exposure). We also assessed interaction effects between chemicals from different chemical groups (DDT/E vs. pyrethroid metabolites).
Analyses were performed with R (3.2.4 Revised [2016-03-16]) or Stata versions 13 and 14 (College Station, TX). BKMR was implemented with the R package bkmr (version 0.1.0).
3. Results
3.1. Participant characteristics
The characteristics of the mothers and children are summarized in Table 1. Mothers were all black South African with a median age of 25 years, low educational attainment (>50% with <12 years of education), nulliparous (57%), and non-smoking (only two reported having ever smoked during pregnancy). Of the 708 infants whose weight and/or height/length were measured at least once, 365 (52%) were male and 343 (48%) were female; 13% (n=92) were born preterm (<37 weeks gestation); and 13% (n=93) were HIV positive. A substantial proportion of households were classified as food insecure (43%) during pregnancy (mean Food Insecurity Index = 1.5; range=0-6) (Table 1). Median child age at the 1-year visit was 372 days and median child age at the 2-year visit was 736 days. Median child body weight was 3135g at birth, 9.0kg at the 1-year visit and 11.2kg at the 2-year visit. Median child BMI was 16.9 (kg/m2) at the 1-year visit and 16.5 (kg/m2) at the 2-year visit. Underweight and overweight prevalence was 4.3% (n=30) and 5.9% (n=41) at the 1-year visit, respectively, while underweight and overweight lowered to 1.6% (n=11) and 4.3% (n=29) as the 2-year visit, respectively.
Table 1.
Child, mother, and household characteristics of VHEMBE study participants with a completed weight measurement at the 1 and/or 2-year visits between 2012 and 2015, Limpopo, South Africa (N=708).
| Variable | N (%) | Mean (±SD) |
|---|---|---|
|
Child Characteristics
| ||
| Sex | ||
|
|
||
| Male | 365 (51.6) | |
|
|
||
| Female | 343 (48.4) | |
|
|
||
| Age (days) | ||
|
|
||
| At 1-year visit | 372 (26) | |
|
|
||
| At 2-year visit | 736 (21) | |
|
|
||
| Gestation | ||
|
|
||
| Age (weeks) | 39 (2) | |
|
|
||
| Preterm (<37 weeks) | 92 (13.0) | |
|
|
||
| Full term (>=37 weeks) | 616 (87.0) | |
|
|
||
| Body weight and length at birth | ||
|
|
||
| Weight (g) | 3135 (447) | |
|
|
||
| Weight (z-score) | −0.51 (1.19) | |
|
|
||
| Length (cm) | 48.9 (2.3) | |
|
|
||
| Length (z-score) | −0.58 (1.13) | |
|
|
||
| Body weight, length, and composition at 1-year visit | ||
|
|
||
| Weight (kg) | 9.00 (1.34) | |
|
|
||
| Weight-for-age (z-score) | −0.43 (1.23) | |
|
|
||
| Weight-for-length (z-score) | 0.001 (1.23) | |
|
|
||
| Length (cm) | 73.0 (2.9) | |
|
|
||
| Length-for-age (z-score) | −0.89 (1.13) | |
|
|
||
| BMI (kg/m2) | 16.9 (1.8) | |
|
|
||
| BMI (z-score) | 0.12 (1.22) | |
|
|
||
| Underweight | 30 (4.3) | |
|
|
||
| Overweight | 41 (5.9) | |
|
|
||
| Body weight, length, and composition at 2-year visit | ||
|
|
||
| Weight (kg) | 11.20 (1.33) | |
|
|
||
| Weight-for-age (z-score) | −0.55 (0.99) | |
|
|
||
| Weight-for-height (z-score) | 0.20 (1.01) | |
|
|
||
| Height (cm) | 82.3 (3.16) | |
|
|
||
| Height (z-score) | −1.50 (0.99) | |
|
|
||
| BMI (kg/m2) | 16.5 (1.42) | |
|
|
||
| BMI (z-score) | 0.55 (1.03) | |
|
|
||
| Underweight | 11 (1.6) | |
|
|
||
| Overweight | 29 (4.3) | |
|
|
||
| Breast feeding | ||
|
|
||
| Duration (months) | 14.7 (7.9) | |
|
Maternal Characteristics | ||
| Age (years) | 26 (6) | |
|
|
||
| Weight (kg, post-partum) | 68.8 (13.7) | |
|
|
||
| Height (cm) | 158.2 (6.8) | |
|
|
||
| BMI (kg/m2, post-partum) | 27.5 (5.4) | |
|
|
||
| HIV Status | ||
|
|
||
| HIV + | 93 (13.2) | |
|
|
||
| HIV − | 612 (86.8) | |
|
|
||
| Parity | ||
|
|
||
| 0 previous births | 309 (43.6) | |
|
|
||
| 1+ previous births | 399 (56.4) | |
|
|
||
| Educational attainment | ||
|
|
||
| <12 grade | 391 (55.2) | |
|
|
||
| Grade 12 | 213 (30.1) | |
|
|
||
| Further studies started | 47 (6.6) | |
|
|
||
| Diploma or further degree | 57 (8.1) | |
|
|
||
| Smoked during pregnancy | ||
|
|
||
| Yes | 3 (0.4) | |
|
|
||
| No | 705 (99.6) | |
|
Household Characteristics | ||
| Income (Rand) | ||
|
|
||
| At birth | 3127 (3760) | |
|
|
||
| 1-year visit | 3893 (4689) | |
|
|
||
| 2-year visit | 4710 (5095) | |
|
|
||
| Below the Food Poverty Line | ||
|
|
||
| Yes | 428 (60.6) | |
|
|
||
| No | 277 (39.4) | |
|
|
||
| Food Security Index | ||
|
|
||
| At birth | 1.6 (1.8) | |
|
|
||
| 1-year visit | 1.6 (1.9) | |
|
|
||
| 2-year visit | 1.4 (1.8) | |
3.2. Prenatal insecticide exposure
Summary statistics for each insecticide biomarker of exposure measured from maternal blood and urine are shown in Table 2. The serum lipid-adjusted geometric means (GMs) for p,p′-DDT and p,p′-DDE were 68.94 ng/g and 285.52 ng/g, respectively, with p,p′-DDT detected in 98% of mothers and p,p′-DDE in all samples. Each of the four urinary pyrethroid metabolites was detected in all mothers, with the exception of one sample for 3-PBA. The specific gravity-standardized GMs for cis-DBCA, cis-DCCA, trans-DCCA, and 3-PBA were 0.22 μg/L, 0.31 μg/L, 0.36 μg/L, and 0.71 μg/L, respectively. There was practically no correlation between concentrations of any of the urinary pyrethroids with the levels of serum p,p′-DDT and p,p′-DDE (ρ range: −0.0003, 0.047); however, correlations within the pyrethroids (ρ range: 0.46 to 0.88) and within the DDT/E measures (ρ=0.86) were moderate to high (Figure S2, Supplemental Material).
3.3. Association between prenatal DDT/E and child body weight and body composition
As shown in Table 3, the adjusted linear mixed effects regression for associations between p,p′-DDT or p,p′-DDE and body weight and body composition measures were overall in the positive direction, but were not statistically significant. However, when we stratified by sex (Table 3), we observed non-significant negative associations for boys but statistically significant positive associations for girls between p,p′-DDT and weight-for-age (adjusted (a)β=0.18, 95% CI= 0.06, 0.31; p-interaction_sex=0.01), BMI-for-age (aβ =0.24, 95% CI= 0.12, 0.36; p-interaction_sex=0.001), and weight-for-height z-scores (aβ=0.23, 95% CI= 0.11, 0.35; p-interaction_sex=0.002) (Table 3). Similarly, associations for p,p′-DDE were negative albeit non-significant for boys but statistically significantly positive for girls for weight-for-age (aβ=0.18, 95% CI= 0.02, 0.32; p-interaction_sex=0.13), BMI-forage (aβ=0.20, 95% CI= 0.05, 0.35; p-interaction_sex=0.02), and weight-for-height z-scores (aβ=0.20, 95% CI= 0.05, 0.34; p-interaction_sex=0.03). The GAM models for p,p′-DDT and BMI-for-age and weight-for-height in girls suggested possible non-linearity; however, this would not have biased our inference in terms of the direction of the association (see Supplemental Figure S3). We did not observe interaction by maternal energy intake or HIV status, household food insecurity or household food poverty. A sensitivity analysis, using the DDT and DDE values without lipid adjustment, but instead fitting lipids as a covariate in the model, did not substantively alter our findings (data not shown).
Table 3.
Overall and sex-stratified adjusted random effects linear single pollutant models for repeated measures of child body weight and body composition in VHEMBE children at 1 and 2 years of age.
| Insecticide Exposure | Outcome |
Overalla
|
Girlsb
|
Boysc
|
|||
|---|---|---|---|---|---|---|---|
| β (95% CI)e | p | β (95% CI) e | p | β (95% CI) e | p | ||
|
p,p′-DDT
| |||||||
| Weight-for-aged | 0.071 (−0.021, 0.162) | 0.13 | 0.183 (0.061, 0.305)* | 0.004 | −0.036 (−0.168, 0.096)* | 0.59 | |
| BMI-for-aged | 0.077 (−0.013, 0.168) | 0.10 | 0.239 (0.116, 0.361)* | <0.001 | −0.055 (−0.185, 0.075)* | 0.41 | |
| Weight-for-heightd | 0.078 (−0.012, 0.168) | 0.09 | 0.229 (0.107, 0.351)* | <0.001 | −0.051 (−0.181, 0.079)* | 0.45 | |
|
p,p′-DDE | |||||||
| Weight-for-aged | 0.071 (−0.039, 0.182) | 0.21 | 0.178 (0.031, 0.325) | 0.02 | −0.029 (−0.190, 0.132) | 0.73 | |
| BMI-for-aged | 0.041 (−0.068, 0.151) | 0.46 | 0.196 (0.047, 0.345)* | 0.01 | −0.083 (−0.241, 0.076)* | 0.31 | |
| Weight-for-heightd | 0.050 (−0.059, 0.159) | 0.37 | 0.195 (0.048, 0.343)* | 0.01 | −0.051 (−0.181, 0.079)* | 0.45 | |
N=692
N=334
N=358
Adjusted for maternal BMI, maternal education, maternal age, maternal parity, household poverty status, and maternal HIV status.
β interpreted as a per 10-fold increase in maternal prenatal organochlorine concentration (ng/g, lipid-adjusted).
Sex interaction is statistically significant at p-value <0.1.
3.4. Association between maternal pyrethroid concentrations and child body weight and body composition
Table 4 presents the results from the adjusted linear mixed effects regression for associations between the maternal urinary concentrations of the pyrethroid metabolites and body weight and body composition measures. Overall, cis-DBCA was significantly associated with lower BMI-for-age (aβ= −0.16 [95%CI: −0.30, −0.02], p=0.03) and weight-for-height (aβ= −0.16 [95%CI: −0.30, −0.02], p=0.03), and marginally associated with lower weight-for-age (aβ= −0.14 [95%CI: −0.28, 0.01], p=0.06) (Table 4) and trans-DCCA was also associated with a lower BMI-for-age (aβ= −0.17 [95%CI: −0.31, −0.03], p=0.02) and weight-for-height (aβ= −0.16 [95%CI: −0.30, −0.02], p=0.03), but not weight-for-age (Table 4). For each pyrethroid metabolite, there were important differences by sex, with significant negative associations seen in boys only. In girls, associations were considerably weaker compared to associations in boys and there was no consistent pattern in pyrethroid associations among girls. This was most prominent for trans-DCCA and cis-DBCA, where we consistently observed statistically significant or marginally significant negative associations among boys in weight-for-age (ptrans-DCCA=0.05; pcis-DBCA=0.06), BMI-for-age (ptrans-DCCA=0.04; pcis-DBCA=0.05), and weight-for-height (ptrans-DCCA=0.03; pcis-DBCA=0.05) but no significant associations among girls (Table 4). In addition, the GAMs suggested non-linear relationships for some of the pyrethroid metabolites, particularly among girls. Plots of the smooth terms for several of these pyrethroids (cis- and trans-DCCA and 3PBA) exhibited a J-shaped (quadratic) curve in relationship to weight-for-age in girls (Supplemental Figure S4). Similar to DDT/E associations, we only observed evidence for interaction by sex and not by other maternal (e.g., HIV) or household factors (e.g., food poverty).
Table 4.
Overall and sex-stratified adjusted random effects linear single pollutant models for repeated measures of child body weight and body composition in VHEMBE children at 1 and 2 years of age.
| Insecticide Exposure | Outcome |
Overalla
|
Girlsb
|
Boysc
|
|||
|---|---|---|---|---|---|---|---|
| β (95% CI) | p | β (95% CI) | p | β (95% CI) | p | ||
|
cis-DBCA
| |||||||
| Weight-for-aged | −0.135 (−0.278, 0.007) | 0.06 | −0.063 (−0.252, 0.126) | 0.52 | −0.199 (−0.406, 0.008) | 0.06 | |
| BMI-for-aged | −0.155 (−0.295, −0.015) | 0.03 | −0.097 (−0.289, 0.095) | 0.33 | −0.204 (−0.408, −0.001) | 0.05 | |
| Weight-for-heightd | −0.155 (−0.296, −0.015) | 0.03 | −0.090 (−0.280, 0.101) | 0.36 | −0.211 (−0.415, −0.008) | 0.05 | |
|
cis-DCCA | |||||||
| Weight-for-aged | −0.045 (−0.210, 0.119) | 0.59 | 0.113 (−0.110, 0.336)* | 0.33 | −0.166 (−0.399, 0.067)* | 0.17 | |
| BMI-for-aged | −0.111 (−0.273, 0.051) | 0.18 | −0.012 (−0.239, 0.215) | 0.92 | −0.180 (−0.409, 0.049) | 0.13 | |
| Weight-for-heightd | −0.100 (−0.262, 0.062) | 0.23 | 0.014 (−0.211, 0.239) | 0.90 | −0.182 (−0.411, 0.048) | 0.13 | |
|
trans-DCCA | |||||||
| Weight-for-aged | −0.095 (−0.238, 0.048) | 0.20 | 0.044 (−0.155, 0.243)* | 0.67 | −0.201 (−0.400, −0.002)* | 0.05 | |
| BMI-for-aged | −0.167 (−0.308, −0.026) | 0.02 | −0.099 (−0.301, 0.103) | 0.34 | −0.210 (−0.406, −0.015) | 0.04 | |
| Weight-for-heightd | −0.156 (−0.297, −0.016) | 0.03 | −0.071 (−0.271, 0.130) | 0.50 | −0.216 (−0.411, −0.020) | 0.03 | |
|
3-PBA | |||||||
| Weight-for-aged | −0.071 (−0.244, 0.102) | 0.42 | 0.071 (−0.170, 0.312)* | 0.57 | −0.159 (−0.400, 0.082)* | 0.20 | |
| BMI-for-aged | −0.128 (−0.298, 0.042) | 0.14 | −0.030 (−0.274, 0.214) | 0.81 | −0.187 (−0.424, 0.050) | 0.13 | |
| Weight-for-heightd | −0.120 (−0.290, 0.050) | 0.17 | −0.008 (−0.250, 0.235) | 0.95 | −0.188 (−0.425, 0.050) | 0.13 | |
N=683
N=328
N=355
Adjusted for maternal BMI, maternal education, maternal age, maternal parity, household poverty status, and maternal HIV status
β interpreted as a per 10-fold increase in maternal prenatal pyrethroid metabolite concentration (μg/L, specific-gravity adjusted).
Sex interaction is statistically significant at p-value <0.1.
3.5. Insecticide Mixtures Analysis
3.5.1. Variable importance and exposure-response relationships
The probabilities of inclusion derived from the BKMR model for the two exposure groups (DDT/E and pyrethroids) and individual exposures within each group are summarized in the Supplemental Materials (Table S2). Among girls, the DDT/E group exceeded the 0.5 group-PIP threshold and was higher for all measures than the pyrethroid group (pyrethroids only reached the PIP threshold for weight-for-age). Within the DDT/E group, p,p′-DDT resulted in conditional PIPs that far exceeded our 0.5 probability threshold (conditional PIPDDT=99%), while p,p′-DDE was rarely selected. In the pyrethroid group none of the conditional PIPs reached the 0.5 threshold. Among boys, the pyrethroid group, but not the DDT/E group, exceeded the 0.5 group-PIP threshold. Although no pyrethroid metabolites exceeded the conditional PIP threshold, trans-DCCA and cis-DBCA showed the highest conditional PIPs.
The sex-specific exposure-response relationships for each chemical estimated from BKMR (holding all other exposures at their median levels) are presented graphically in Figure 1. For the most part, the direction of exposure-responses from BKMR were consistent with the associations observed in the single-pollutant linear regression models. However, for p,p′-DDE in girls, the negative exposure-response from the multi-pollutant BKMR analysis was in the opposite direction as the single pollutant model results (Table 3), albeit with a low inclusion probability (PIP) in BKMR (Table S2). We note that when p,p′-DDT is included in the conventional linear regression (although correlation is high; but the Variance Inflation Factor was <4), the association of p,p′-DDE is no longer present but the strong association of p,p′-DDT remains (data not shown).
Figure 1.
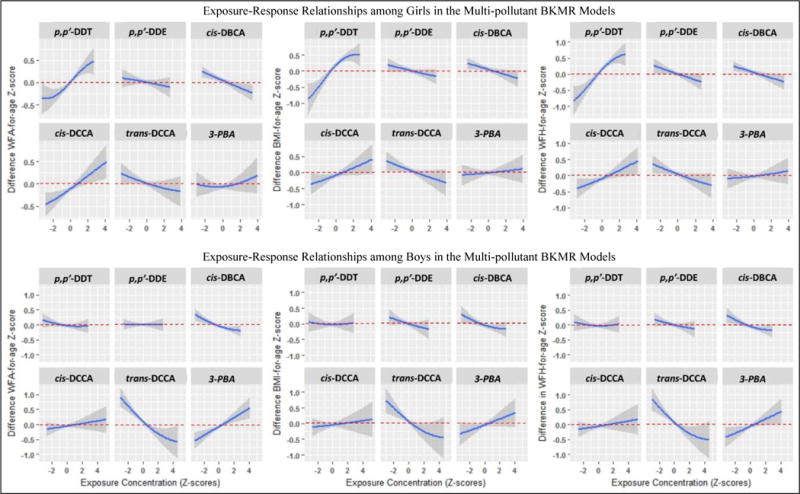
Blue lines represent the exposure-response relationships for individual chemicals from the BKMR analysis. Shaded areas represent 95% credible intervals. We would interpret this as the predicted difference in z-score at a given exposure level (e.g., p,p′-DDT) compared to the median exposure level of that chemical, while holding all other chemical exposures at their median. The top row are results for girls and the bottom row are results for boys.
3.5.2. Combined effects within mixture
Using BKMR, we determined if the association of each individual chemical on body weight and body composition measures differed when all other insecticide exposure measures varied together cumulatively. In Figure 2 we show the variation in the association of an interquartile range [IQR]) increase of each individual insecticide exposure measure, when the other exposures in the mixture were held at their 5th to 95th percentile levels.
Figure 2.
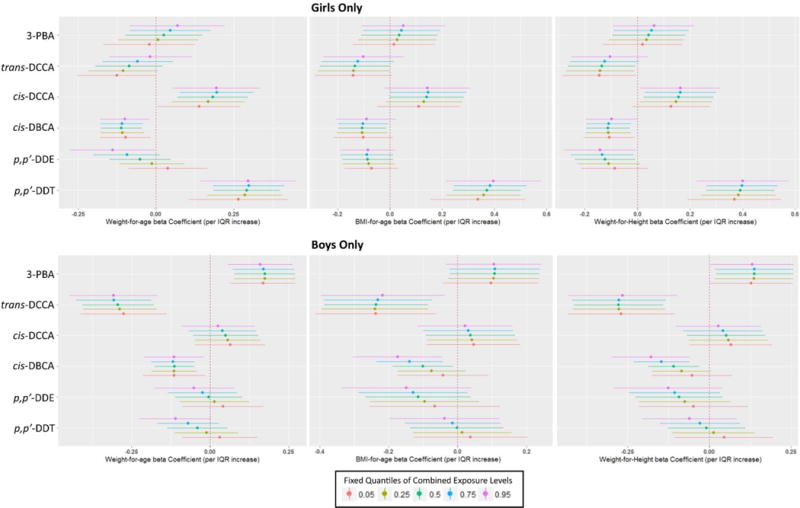
Plots of single-chemical exposure-response relationships (and 95% credible intervals) with all other exposures fixed at quantiles, stratified by outcome and sex from the multipollutant BKMR analyses: (a) Weight-for-age among girls; (b) BMI-for-age among girls; (c) Weight-for-height among girls; (d) Weight-for-age among boys; (e) BMI-for-age among boys; (f) Weight-for-height among boys.
Among girls, the IQR effect estimate for p,p′-DDE on weight-for-age changed from a marginally positive association to a negative one that did not include the null, when comparing the combined exposure at the 5th to the 95th percentile (Figure 2a). For trans-DCCA on weight-for-age in girls, the effect estimates were in the negative direction at the low levels of combined exposure quantiles but the magnitude of the effect estimate attenuated towards the null at higher levels of combined exposure. Several other chemicals showed shifts in magnitude of association in girls but did not alter the direction or strength of association (see Figure 2).
Among boys, we found a substantial change in the effect estimates for cis-DBCA on lower BMI-for-age and weight-for-height between the 5th and 95th percentile combined exposure level, with the 95% credible interval including the null at low combined exposure (5th percentile) but negative associations at higher combined exposure (50th percentile to 95th percentile). A similar trend was seen for p,p′-DDT and weight-for-age among boys, with lower combined exposure showing a slightly positive association for p,p′-DDT that included the null but a negative association that did not include the null at the highest level of combined exposure. A somewhat similar pattern was seen for p,p′-DDE, although the effect estimates included the null at all levels of combined exposure.
The BMKR models also suggested evidence of between-chemical interactions on study outcomes in boys but not in girls. In boys, the negative exposure-response (on BMI-for-age and weight-for-height) for cis-DBCA became steeper at higher levels of p,p′-DDT or p,p′-DDE (Figures S5c and S5d). Conventional linear mixed effects regression models confirmed that these interactions were statistically significant in boys (DDE*cis-DBCA, pinteraction=0.05; DDT*cis-DBCA, pinteraction < 0.10; data not shown).
4. Discussion
In this study we evaluated associations between measures of prenatal exposure to pyrethroids and organochlorines (p,p′-DDT, and p,p′-DDE) and body weight and body composition in children currently exposed in an IRS setting. We also considered associations within the context of co-exposure patterns (or “exposure mixture”). In single-pollutant and multi-pollutant models we found that among girls but not boys, prenatal p,p′-DDT was positively associated with body weight and body composition outcomes. In contrast, among boys but not girls, single-pollutant and multi-pollutant analyses suggested that prenatal pyrethroid exposures, in particular cis-DBCA and trans-DCCA, were significantly associated with lower body composition measures. Several of the pyrethroids were non-linearly related to body weight, particularly in girls. The BKMR models in girls and in boys suggested that the magnitude of chemical exposure-responses for some chemicals were influenced by changes in the overall levels of the exposure mixture. Such mixture effects were most notable for p,p′-DDE (i.e., changed from null to negative associations at very high cumulative exposure) and trans-DCCA (i.e., attenuated the negative association at very high cumulative exposure) on body weight in girls, and for cis-DBCA (i.e., changed from null to negative association) on body composition in boys. BKMR models in boys also showed that as p,p′-DDT and, even more significantly, p,p′-DDE concentrations increased, the negative exposure-response relationship for cis-DBCA on weight-for-age became increasingly negative.
4.1. DDT/E associations with child body weight and body composition
Our consistent findings across the single- and multi-pollutant models of positive associations between maternal p,p′-DDT serum levels and body weight and body composition in girls supports the hypothesis that early-life exposure to certain xenobiotic EDCs may act as obesogens in childhood (Braun, 2016; Cano-Sancho et al., 2017). While in vitro studies show that both p,p′-DDT and p,p′-DDE promote adipocyte cell growth, differentiation and/or dysfunction (Cano-Sancho et al., 2017; Howell and Mangum, 2011; Kim et al., 2016; Moreno-Aliaga and Matsumura, 2002), our findings in humans support only obesogenic associations with p,p′-DDT exposure. Our initial finding from the single-pollutant model of a positive association of p,p′-DDE did not hold in the multi-pollutant BKMR model, or in the conventional linear regression model after controlling for p,p′-DDT. This finding suggests that the initial positive obesogenic associations of p,p′-DDE in girls from conventional regression were possibly spurious due to its high correlation with serum p,p′-DDT levels. The differences in observed effects between p,p′-DDT and p,p′-DDE in our study could plausibly be related to their relative endocrine disruption activity, with p,p′-DDT being a stronger agonist of estrogen (Shekhar et al., 1997) – which is known to be involved in deposition, differentiation, and metabolism of adipose tissue (Pallottini et al., 2008) – and p,p′-DDE showing both anti-androgen and estrogenic activity (Kelce et al., 1995; Sohoni, 1998). Differences in our results from previous epidemiologic studies, which found associations between prenatal p,p′-DDE exposure and increased childhood body composition (Agay-Shay et al., 2015; Delvaux et al., 2014; Heggeseth et al., 2015; Iszatt et al., 2015; La Merrill and Birnbaum, 2011; Lee et al., 2011; Tang-Peronard et al., 2014; Vafeiadi et al., 2015; Valvi et al., 2011, 2015; Verhulst et al., 2009), may be explained in part by the relative differences in p,p′-DDE and p,p′-DDT levels (e.g., due to the recency and level of exposure) and/or by failure to account for DDT co-exposure in the other studies. In addition, the generalizability of previous studies-conducted predominantly in higher-income countries- may be in question because these populations may be co-exposed to other EDCs that our population from a lower-income country is not exposed to. Moreover, we had very low smoking prevalence in the VHEMBE cohort and the relatively lower socioeconomic level of our study participants may explain some of the contrasting findings between our study and previous ones. We note that our finding of no associations for DDE, in multipollutant models, concurs with a number of studies that did not observe an association between prenatal DDE concentrations and child growth or body composition measures (Cupul-Uicab et al., 2010, 2013; Garced et al., 2012; Gladen et al., 2004; Høyer et al., 2014; Karlsen et al., 2016; Tang-Peronard et al., 2014; Warner et al., 2013).
As noted in a recent meta-analysis, there is little consistency in the literature with respect to sex-specific effects of prenatal p,p′-DDE or p,p′-DDT on body composition (Cano-Sancho et al., 2017). While the studies that measured p,p′-DDT in maternal serum are considerably fewer in number compared to those that studied p,p′-DDE only, our finding that maternal p,p′-DDT levels were associated with increased body measurements in girls, but marginally lower body measurements in boys, conflicts with results from two other studies that found positive associations between maternal levels of p,p′-DDT and BMI in boys but not girls (Valvi et al., 2011; Warner et al., 2014). Although both studies (Valvi et al., 2011; Warner et al., 2014) measured prenatal p,p′-DDE in addition to p,p′-DDT, neither study modeled their co-exposures, while one (Warner et al., 2014) reported a null association of p,p′-DDE in both sexes and the other (Valvi et al., 2011) showed a negative association for p,p′-DDE in boys (consistent with our finding). Another study found that prenatal p,p′-DDE was positively associated with body composition in girls but not in boys (Delvaux et al., 2014). Given the limited and conflicting epidemiological evidence and the lack of experimental research on sex-specific obesogenic effects of DDT or DDE in children, it is unclear what precise mechanisms may give rise to sex-specific effects or what could explain the discrepancy between our study and the others that measured DDT (Valvi et al., 2011; Warner et al., 2014). While differences in co-exposure patterns to other EDCs between very different study populations could partially explain the discrepancies, more experimental and epidemiological evidence are clearly needed to elucidate the mechanisms of sex-specific effects of EDCs in general. The role during the fetal period (a critical time for hormones to organize energy balance) of DDT as an environmental estrogen versus DDE as an environmental androgen warrants further investigation. Additionally, the known differences in the deposition, mobilization, and utilization of fat between girls and boys, at different stages of growth and development, should be taken into consideration in experimental studies examining EDC obesogenic effects (Braun, 2016; Heindel et al., 2017).
4.2. Pyrethroid metabolite associations with child body weight and body composition
In our study, trans-DCCA and cis-DBCA were associated with lower BMI-for-age and weight-for-height z-scores for both sexes, but especially among boys. Unfortunately, there are no previous epidemiological studies on pyrethroid exposures and body weight and body composition in childhood. One study conducted in rural Northern China (Ding et al., 2015) on neonates reported that prenatal exposure to multiple pyrethroid metabolites (as measured by the sum of metabolites of cis-DCCA, trans-DCCA, and 3-PBA) was associated with reduced birth weight but not birth length, head circumference, or gestational duration. Despite the paucity of epidemiologic studies, our results on pyrethroids are consistent with experimental evidence of their endocrinologic activity (Brander et al., 2016). An in vitro study (Du et al., 2010) of 11 pyrethroids or metabolites found DCCA to have the strongest anti-estrogenic activity, and deltamethrin and cyfluthrin to have the strongest androgen receptor activity. Deltamethrin (which devolves to cis-DBCA) and cyfluthrin (which devolves to cis-DCCA, trans-DCCA, or 4-fluro-3PBA) are the same parent compounds that precede the metabolites shown in our study to impose the strongest and most consistent negative associations with body weight and body composition measurements in boys. Interestingly, we found significant interaction between cis-DBCA and p,p′-DDE on body composition, and both compounds have been shown to be androgen antagonists (Brander et al., 2016; Du et al., 2010; Kelce et al., 1995; Xu et al., 2006).
4.4. Strengths and Limitations
A major limitation of this study is our exposure assessment of urinary pyrethroids. We sampled single spot-urine from mothers at or just after delivery and given the short half-life of pyrethroids (6.4 - 16.5 hours (ATSDR, 2003)) and within-individual variability, it is uncertain how well such measurements represent average exposure levels throughout the entire pregnancy. Two recent studies (Attfield et al., 2013; Morgan et al., 2016) indicated that a single spot-urine sample does not represent average exposure over time, either over a few days, a week, or an entire year. If this is the case in our study, then our reliance on a single spot-urine sample could have resulted in exposure misclassification and our results would likely have been biased towards the null. We note, however, that in preliminary analyses (data not shown) the maternal pyrethroid metabolite concentrations in our study were significantly related to indicators of longer-term pesticide exposure obtained from questionnaire (e.g., whether the village of maternal residence was sprayed for IRS) and home inspections (pesticides stored in the home). Therefore, it is possible that in our study the spot urine samples may be reflective of long-term exposures. Nevertheless, only future studies that collect multiple urine samples over the course of a pregnancy could more fully address the limitation of this study.
Regarding the organochlorines, given the relatively long half-life of p,p′-DDT and p,p′-DDE, serum levels measured at different trimesters are highly correlated (Longnecker et al., 1999) and it is highly likely that a single measurement around delivery will provide an excellent measure of prenatal exposure. Nevertheless, we did not assess postnatal exposure to either p,p′-DDT, p,p′-DDE, or pyrethroids; postnatal exposures could impose either a dose-additive effect to the prenatal associations or separate postnatal biologic effects that we did not capture in our study.
The pyrethroid metabolite cis-DBCA—specific to deltamethrin and a commonly used IRS insecticide—exhibited one of the strongest and consistent associations in both the single- and multi-pollutant models. However, the other pyrethroid metabolites (e.g., cis-DCCA, trans-DCCA, and 3-PBA) are non-specific to a parent pyrethroid compound, which limits our ability to attribute specific pyrethroids as a causative agent.
Another important limitation, with respect to the BKMR analyses, is the lack of occurrence of co-exposure patterns with high DDT and low DDE, or vice-versa. Similarly, there was a lack of occurrence of co-exposure patterns with high cis-DCCA, low trans-DCCA, and low 3-PBA, or vice-versa. Such co-exposure patterns entail that the BKMR “mixtures effect” estimates rely on some degree of extrapolation of the data. However, our emphasis on interaction effects between chemical classes of insecticides (organochlorines versus pyrethroids), as opposed to within classes, was done in acknowledgement of the inherent limitations with the co-exposure patterns in our cohort.
Our study also had certain strengths. The major strength of this study is the uniqueness of the population as it relates to investigating co-exposure to multiple insecticides and associations with body weight and body composition in toddlers, particularly because the cohort lives in an area where these chemicals are actively in use and because this is a relatively low socioeconomic status setting. The recency of likely DDT exposures in combination with pyrethroids, due to active IRS campaigns, makes for distinct exposure profiles that are otherwise not readily studied elsewhere. In addition, the level of serum DDT was strikingly higher compared to previous studies in higher-income countries studying effects of DDT on child body composition. For instance, the GM maternal serum levels of p,p′-DDT (lipid-adjusted) observed in the VHEMBE cohort was more five-times higher compared to the levels we observed in the CHAMACOS cohort from the U.S (Warner et al., 2013). Serum GM p,p′-DDT levels (unadjusted) in the our cohort (VHEMBE) was six-times higher than a study in a Spanish cohort (Valvi et al., 2011). Another important strength is our sampling procedures and statistical analyses with BKMR. Our approach enabled us to examine joint exposure to these insecticides and their associations with body weight and body composition measured longitudinally. Although with some limitations noted above, BKMR models allowed us to study complex chemical mixture effects not easily explored using conventional regression modeling alone.
5.0. Conclusion
In a longitudinal South African birth cohort of more than 600 children age 2 years or less, we observed significant sex-specific associations between early-life developmental insecticide exposures with childhood BMI and weight. Our analyses provide evidence that prenatal exposure to DDT used for IRS in South Africa could potentially play a role in the growing obesity epidemic among South African girls (Pienaar, 2015; Sartorius et al., 2015). Conversely, our analysis provides evidence that pyrethroid exposure used in IRS could play a role in the increasingly higher rates of underweight noted in South African boys (Kimani-Murage et al., 2010; Kruger, 2014). Given the paucity of data available on child health and pyrethroid exposure, especially in populations where IRS is currently applied, additional epidemiology studies in this area are critically needed. Our findings also underscore the need for future studies to consider the effects of chemical mixtures.
Supplementary Material
Highlights.
p,p′-DDT was associated with higher body composition in girls only
Pyrethroid metabolites were associated with lower body composition in boys
Insecticide exposure associations were influenced by the overall exposure mixture
Acknowledgments
This research was funded by the National Institute of Environmental Health Sciences (Award ID: 1R01ES020360), the Health Resources and Services Administration, Maternal and Child Health Bureau (Award ID: T76MC00002), and the Global Health Equity Scholars Program, Fogarty TW009338.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
All authors report no competing interests.
References
- Agay-Shay K, Martinez D, Valvi D, Garcia-Esteban R, Basagaña X, Robinson O, Casas M, Sunyer J, Vrijheid M. Exposure to Endocrine-Disrupting Chemicals during Pregnancy and Weight at 7 Years of Age: A Multi-pollutant Approach. Environ Health Perspect. 2015;123:1030–1037. doi: 10.1289/ehp.1409049. https://doi.org/10.1289/ehp.1409049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agier L, Portengen L, Chadeau-Hyam M, Basagaña X, Giorgis-Allemand L, Siroux V, Robinson O, Vlaanderen J, González JR, Nieuwenhuijsen MJ, Vineis P, Vrijheid M, Slama R, Vermeulen R. A Systematic Comparison of Linear Regression–Based Statistical Methods to Assess Exposome-Health Associations. Environ Health Perspect. 2016;124 doi: 10.1289/EHP172. https://doi.org/10.1289/EHP172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ATSDR. Toxicological Profile for Pyrethrins and Pyrethroids. US Department of Health and Human Services, Agency for Toxic Substances and Disease Registry; Atlanta, GA, USA: 2003. [PubMed] [Google Scholar]
- Attfield KR, Hughes MD, Spengler JD, Lu C (Alex) Within- and Between-Child Variation in Repeated Urinary Pesticide Metabolite Measurements over a 1-Year Period. Environ Health Perspect. 2013 doi: 10.1289/ehp.1306737. https://doi.org/10.1289/ehp.1306737. [DOI] [PMC free article] [PubMed]
- Barr JR, Maggio VL, Barr DB, Turner WE, Sjödin A, Sandau CD, Pirkle JL, Needham LL, Patterson DG. New high-resolution mass spectrometric approach for the measurement of polychlorinated biphenyls and organochlorine pesticides in human serum. J Chromatogr B Analyt Technol Biomed Life Sci. 2003;794:137–148. doi: 10.1016/s1570-0232(03)00451-3. [DOI] [PubMed] [Google Scholar]
- Berger JO, Barbieri MM. Optimal predictive model selection. Ann Stat. 2004;32:870–897. https://doi.org/10.1214/009053604000000238. [Google Scholar]
- Bobb JF, Valeri L, Claus Henn B, Christiani DC, Wright RO, Mazumdar M, Godleski JJ, Coull BA. Bayesian kernel machine regression for estimating the health effects of multi-pollutant mixtures. Biostatistics. 2015;16:493–508. doi: 10.1093/biostatistics/kxu058. https://doi.org/10.1093/biostatistics/kxu058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bobb JF, Wellenius GA, Mittleman MA, Deaco B, Coull BA. Bayesian kernel machine regression for estimating the health effects of multi-pollutant mixtures. 2013 doi: 10.1093/biostatistics/kxu058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouwman H, Sereda B, Meinhardt HM. Simultaneous presence of DDT and pyrethroid residues in human breast milk from a malaria endemic area in South Africa. Environ Pollut. 2006;144:902–917. doi: 10.1016/j.envpol.2006.02.002. https://doi.org/10.1016/j.envpol.2006.02.002. [DOI] [PubMed] [Google Scholar]
- Brander SM, Gabler MK, Fowler NL, Connon RE, Schlenk D. Pyrethroid Pesticides as Endocrine Disruptors: Molecular Mechanisms in Vertebrates with a Focus on Fishes. Environ Sci Technol. 2016;50:8977–8992. doi: 10.1021/acs.est.6b02253. https://doi.org/10.1021/acs.est.6b02253. [DOI] [PubMed] [Google Scholar]
- Braun JM. Early-life exposure to EDCs: role in childhood obesity and neurodevelopment. Nat Rev Endocrinol. 2016 doi: 10.1038/nrendo.2016.186. https://doi.org/10.1038/nrendo.2016.186. [DOI] [PMC free article] [PubMed]
- Cano-Sancho G, Salmon AG, La Merrill MA. Association between Exposure to p, p′-DDT and Its Metabolite p, p′-DDE with Obesity: Integrated Systematic Review and Meta-Analysis. Environ Health Perspect. 2017;125 doi: 10.1289/EHP527. https://doi.org/10.1289/EHP527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevalier N, Fénichel P. Obésité, diabète de type 2 et perturbateurs endocriniens. Presse Médicale. 2016;45:88–97. doi: 10.1016/j.lpm.2015.08.008. https://doi.org/10.1016/j.lpm.2015.08.008. [DOI] [PubMed] [Google Scholar]
- Coker E, Gunier R, Bradman A, Harley K, Kogut K, Molitor J, Eskenazi B. Association between Pesticide Profiles Used on Agricultural Fields near Maternal Residences during Pregnancy and IQ at Age 7 Years. Int J Environ Res Public Health. 2017;14:506. doi: 10.3390/ijerph14050506. https://doi.org/10.3390/ijerph14050506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coull BA, Bobb JF, Wellenius GA, Kioumourtzoglou MA, Mittleman MA, Koutrakis P, Godleski JJ. Part 1. Statistical Learning Methods for the Effects of Multiple Air Pollution Constituents. Res Rep Health Eff Inst. 2015:5–50. [PubMed] [Google Scholar]
- Cupul-Uicab LA, Hernández-Ávila M, Terrazas-Medina EA, Pennell ML, Longnecker MP. Prenatal exposure to the major DDT metabolite 1,1-dichloro-2,2-bis(p-chlorophenyl)ethylene (DDE) and growth in boys from Mexico. Environ Res. 2010;110:595–603. doi: 10.1016/j.envres.2010.06.001. https://doi.org/10.1016/j.envres.2010.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cupul-Uicab LA, Klebanoff MA, Brock JW, Longnecker MP. Prenatal Exposure to Persistent Organochlorines and Childhood Obesity in the U.S. Collaborative Perinatal Project. Environ Health Perspect. 2013 doi: 10.1289/ehp.1205901. https://doi.org/10.1289/ehp.1205901. [DOI] [PMC free article] [PubMed]
- de Cock M, de Boer M, Lamoree M, Legler J, van de Bor M. First Year Growth in Relation to Prenatal Exposure to Endocrine Disruptors — A Dutch Prospective Cohort Study. Int J Environ Res Public Health. 2014;11:7001–7021. doi: 10.3390/ijerph110707001. https://doi.org/10.3390/ijerph110707001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delvaux I, Van Cauwenberghe J, Den Hond E, Schoeters G, Govarts E, Nelen V, Baeyens W, Van Larebeke N, Sioen I. Prenatal exposure to environmental contaminants and body composition at age 7–9 years. Environ Res. 2014;132:24–32. doi: 10.1016/j.envres.2014.03.019. https://doi.org/10.1016/j.envres.2014.03.019. [DOI] [PubMed] [Google Scholar]
- Dewailly E, Forde M, Robertson L, Kaddar N, Laouan Sidi EA, Côté S, Gaudreau E, Drescher O, Ayotte P. Evaluation of pyrethroid exposures in pregnant women from 10 Caribbean countries. Environ Int. 2014;63:201–206. doi: 10.1016/j.envint.2013.11.014. https://doi.org/10.1016/j.envint.2013.11.014. [DOI] [PubMed] [Google Scholar]
- Ding G, Cui C, Chen L, Gao Y, Zhou Y, Shi R, Tian Y. Prenatal exposure to pyrethroid insecticides and birth outcomes in Rural Northern China. J Expo Sci Environ Epidemiol. 2015;25:264–270. doi: 10.1038/jes.2014.86. https://doi.org/10.1038/jes.2014.86. [DOI] [PubMed] [Google Scholar]
- Du G, Shen O, Sun H, Fei J, Lu C, Song L, Xia Y, Wang S, Wang X. Assessing Hormone Receptor Activities of Pyrethroid Insecticides and Their Metabolites in Reporter Gene Assays. Toxicol Sci. 2010;116:58–66. doi: 10.1093/toxsci/kfq120. https://doi.org/10.1093/toxsci/kfq120. [DOI] [PubMed] [Google Scholar]
- Garced S, Torres-Sánchez L, Cebrián ME, Claudio L, López-Carrillo L. Prenatal dichlorodiphenyldichloroethylene (DDE) exposure and child growth during the first year of life. Environ Res. 2012;113:58–62. doi: 10.1016/j.envres.2011.12.002. https://doi.org/10.1016/j.envres.2011.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaspar FW, Chevrier J, Bornman R, Crause M, Obida M, Barr DB, Bradman A, Bouwman H, Eskenazi B. Undisturbed dust as a metric of long-term indoor insecticide exposure: Residential DDT contamination from indoor residual spraying and its association with serum levels in the VHEMBE cohort. Environ Int. 2015;85:163–167. doi: 10.1016/j.envint.2015.09.014. https://doi.org/10.1016/j.envint.2015.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaspar FW, Chevrier J, Quirós-Alcalá L, Lipsitt JM, Barr DB, Holland N, Bornman R, Eskenazi B. Levels and Determinants of DDT and DDE Exposure in the VHEMBE Cohort. Environ Health Perspect. 2017;125 doi: 10.1289/EHP353. https://doi.org/10.1289/EHP353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gladen BC, Klebanoff MA, Hediger ML, Katz SH, Barr DB, Davis MD, Longnecker MP. Prenatal DDT Exposure in Relation to Anthropometric and Pubertal Measures in Adolescent Males. Environ Health Perspect. 2004;112:1761–1767. doi: 10.1289/ehp.7287. https://doi.org/10.1289/ehp.7287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heggeseth B, Harley K, Warner M, Jewell N, Eskenazi B. Detecting Associations between Early-Life DDT Exposures and Childhood Growth Patterns: A Novel Statistical Approach. PLOS ONE. 2015;10:e0131443. doi: 10.1371/journal.pone.0131443. https://doi.org/10.1371/journal.pone.0131443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heindel JJ, Blumberg B, Cave M, Machtinger R, Mantovani A, Mendez MA, Nadal A, Palanza P, Panzica G, Sargis R, Vandenberg LN, vom Saal F. Metabolism disrupting chemicals and metabolic disorders. Reprod Toxicol. 2017;68:3–33. doi: 10.1016/j.reprotox.2016.10.001. https://doi.org/10.1016/j.reprotox.2016.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell G, Mangum L. Exposure to bioaccumulative organochlorine compounds alters adipogenesis, fatty acid uptake, and adipokine production in NIH3T3-L1 cells. Toxicol Vitro Int J Publ Assoc BIBRA. 2011;25:394–402. doi: 10.1016/j.tiv.2010.10.015. https://doi.org/10.1016/j.tiv.2010.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Høyer BB, Ramlau-Hansen CH, Henriksen TB, Pedersen HS, Góralczyk K, Zviezdai V, Jönsson BAG, Heederik D, Lenters V, Vermeulen R, Peter Bonde J, Toft G. Body mass index in young school age children in relation to organochlorine compounds in early life: a prospective study. Int J Obes. 2014 doi: 10.1038/ijo.2014.58. https://doi.org/10.1038/ijo.2014.58. [DOI] [PubMed]
- Iszatt N, Stigum H, Verner MA, White RA, Govarts E, Palkovicova Murinova L, Schoeters G, Trnovec T, Legler J, Pelé F, Botton J, Chevrier C, Wittsiepe J, Ranft U, Vandentorren S, Kasper-Sonnenberg M, Klümper C, Weisglas-Kuperus N, Polder A, Eggesbø M. Prenatal and Postnatal Exposure to Persistent Organic Pollutants and Infant Growth: A Pooled Analysis of Seven European Birth Cohorts. Environ Health Perspect. 2015 doi: 10.1289/ehp.1308005. https://doi.org/10.1289/ehp.1308005. [DOI] [PMC free article] [PubMed]
- Karlsen M, Grandjean P, Weihe P, Steuerwald U, Oulhote Y, Valvi D. Early-life exposures to persistent organic pollutants in relation to overweight in preschool children. Reprod Toxicol. 2016 doi: 10.1016/j.reprotox.2016.08.002. https://doi.org/10.1016/j.reprotox.2016.08.002. [DOI] [PMC free article] [PubMed]
- Kelce WR, Stone CR, Laws SC, Gray LE, Kemppainen JA, Wilson EM. Persistent DDT metabolite p,p′–DDE is a potent androgen receptor antagonist. Nature. 1995;375:581–585. doi: 10.1038/375581a0. https://doi.org/10.1038/375581a0. [DOI] [PubMed] [Google Scholar]
- Kim J, Sun Q, Yue Y, Yoon KS, Whang KY, Marshall Clark J, Park Y. 4,4′-Dichlorodiphenyltrichloroethane (DDT) and 4,4′-dichlorodiphenyldichloroethylene (DDE) promote adipogenesis in 3T3-L1 adipocyte cell culture. Pestic Biochem Physiol. 2016;131:40–45. doi: 10.1016/j.pestbp.2016.01.005. https://doi.org/10.1016/j.pestbp.2016.01.005. [DOI] [PubMed] [Google Scholar]
- Kimani-Murage EW, Kahn K, Pettifor JM, Tollman SM, Dunger DB, Gómez-Olivé XF, Norris SA. The prevalence of stunting, overweight and obesity, and metabolic disease risk in rural South African children. BMC Public Health. 2010;10 doi: 10.1186/1471-2458-10-158. https://doi.org/10.1186/1471-2458-10-158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruger S. The double burden of underweight and overweight in South African adolescents: a challenge and an opportunity for nutritionists and dietitians. South Afr J Clin Nutr. 2014;27:5–6. https://doi.org/10.1080/16070658.2014.11734477. [Google Scholar]
- Kwon D, Landi MT, Vannucci M, Issaq HJ, Prieto D, Pfeiffer RM. An efficient stochastic search for Bayesian variable selection with high-dimensional correlated predictors. Comput Stat Data Anal. 2011;55:2807–2818. doi: 10.1016/j.csda.2011.04.019. https://doi.org/10.1016/j.csda.2011.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Merrill M, Birnbaum LS. Childhood obesity and environmental chemicals. Mt Sinai J Med N Y. 2011;78:22–48. doi: 10.1002/msj.20229. https://doi.org/10.1002/msj.20229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee DH, Steffes MW, Sjödin A, Jones RS, Needham LL, Jacobs DR. Low Dose Organochlorine Pesticides and Polychlorinated Biphenyls Predict Obesity, Dyslipidemia, and Insulin Resistance among People Free of Diabetes. PLoS ONE. 2011;6:e15977. doi: 10.1371/journal.pone.0015977. https://doi.org/10.1371/journal.pone.0015977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longnecker MP, Klebanoff MA, Gladen BC, Berendes HW. Serial levels of serum organochlorines during pregnancy and postpartum. Arch Environ Health. 1999;54:110–114. doi: 10.1080/00039899909602244. https://doi.org/10.1080/00039899909602244. [DOI] [PubMed] [Google Scholar]
- Lubin JH, Colt JS, Camann D, Davis S, Cerhan JR, Severson RK, Bernstein L, Hartge P. Epidemiologic Evaluation of Measurement Data in the Presence of Detection Limits. Environ Health Perspect. 2004;112:1691–1696. doi: 10.1289/ehp.7199. https://doi.org/10.1289/ehp.7199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maharaj R, Moonasar D, Baltazar C, Kunene S, Morris N. Sustaining control: lessons from the Lubombo spatial development initiative in southern Africa. Malar J. 2016;15 doi: 10.1186/s12936-016-1453-9. https://doi.org/10.1186/s12936-016-1453-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meeker JD, Altshul L, Hauser R. Serum PCBs, p,p′-DDE and HCB predict thyroid hormone levels in men. Environ Res. 2007;104:296–304. doi: 10.1016/j.envres.2006.11.007. https://doi.org/10.1016/j.envres.2006.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno-Aliaga MJ, Matsumura F. Effects of 1,1,1-trichloro-2,2-bis(p-chlorophenyl)-ethane (p,p′-DDT) on 3T3-L1 and 3T3-F442A adipocyte differentiation. Biochem Pharmacol. 2002;63:997–1007. doi: 10.1016/s0006-2952(01)00933-9. [DOI] [PubMed] [Google Scholar]
- Morgan MK, Sobus JR, Barr DB, Croghan CW, Chen FL, Walker R, Alston L, Andersen E, Clifton MS. Temporal variability of pyrethroid metabolite levels in bedtime, morning, and 24-h urine samples for 50 adults in North Carolina. Environ Res. 2016;144:81–91. doi: 10.1016/j.envres.2015.11.003. https://doi.org/10.1016/j.envres.2015.11.003. [DOI] [PubMed] [Google Scholar]
- O’Hara RB, Sillanpää MJ. A review of Bayesian variable selection methods: what, how and which. Bayesian Anal. 2009;4:85–117. https://doi.org/10.1214/09-BA403. [Google Scholar]
- Otten JJ, Hellwig JP, Meyers LD, editors. DRI, dietary reference intakes: the essential guide to nutrient requirements. National Academies Press; Washington, DC: 2006. [Google Scholar]
- Pallottini V, Bulzomi P, Galluzzo P, Martini C, Marino M. Estrogen regulation of adipose tissue functions: involvement of estrogen receptor isoforms. Infect Disord Drug Targets. 2008;8:52–60. doi: 10.2174/187152608784139631. [DOI] [PubMed] [Google Scholar]
- Phillips DL, Pirkle JL, Burse VW, Bernert JT, Henderson LO, Needham LL. Chlorinated hydrocarbon levels in human serum: effects of fasting and feeding. Arch Environ Contam Toxicol. 1989;18:495–500. doi: 10.1007/BF01055015. [DOI] [PubMed] [Google Scholar]
- Pienaar AE. Prevalence of overweight and obesity among primary school children in a developing country: NW-CHILD longitudinal data of 6–9-yr-old children in South Africa. BMC Obes. 2015;2:2. doi: 10.1186/s40608-014-0030-4. https://doi.org/10.1186/s40608-014-0030-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sartorius B, Veerman LJ, Manyema M, Chola L, Hofman K. Determinants of Obesity and Associated Population Attributability, South Africa: Empirical Evidence from a National Panel Survey, 2008–2012. PLOS ONE. 2015;10:e0130218. doi: 10.1371/journal.pone.0130218. https://doi.org/10.1371/journal.pone.0130218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott JG, Berger JO. Bayes and empirical-Bayes multiplicity adjustment in the variable-selection problem. Ann Stat. 2010;38:2587–2619. https://doi.org/10.1214/10-AOS792. [Google Scholar]
- Shekhar PVM, Werdell J, Basrur VS. Environmental Estrogen Stimulation of Growth and Estrogen Receptor Function in Preneoplastic and Cancerous Human Breast Cell Lines. JNCI J Natl Cancer Inst. 1997;89:1774–1782. doi: 10.1093/jnci/89.23.1774. https://doi.org/10.1093/jnci/89.23.1774. [DOI] [PubMed] [Google Scholar]
- Sohoni P. Several environmental oestrogens are also anti-androgens. J Endocrinol. 1998;158:327–339. doi: 10.1677/joe.0.1580327. https://doi.org/10.1677/joe.0.1580327. [DOI] [PubMed] [Google Scholar]
- Tang-Peronard JL, Heitmann BL, Andersen HR, Steuerwald U, Grandjean P, Weihe P, Jensen TK. Association between prenatal polychlorinated biphenyl exposure and obesity development at ages 5 and 7 y: a prospective cohort study of 656 children from the Faroe Islands. Am J Clin Nutr. 2014;99:5–13. doi: 10.3945/ajcn.113.066720. https://doi.org/10.3945/ajcn.113.066720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vafeiadi M, Georgiou V, Chalkiadaki G, Rantakokko P, Kiviranta H, Karachaliou M, Fthenou E, Venihaki M, Sarri K, Vassilaki M, Kyrtopoulos SA, Oken E, Kogevinas M, Chatzi L. Association of Prenatal Exposure to Persistent Organic Pollutants with Obesity and Cardiometabolic Traits in Early Childhood: The Rhea Mother–Child Cohort (Crete, Greece) Environ Health Perspect. 2015;123 doi: 10.1289/ehp.1409062. https://doi.org/10.1289/ehp.1409062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valeri L, Mazumdar MM, Bobb JF, Claus Henn B, Rodrigues E, Sharif OIA, Kile ML, Quamruzzaman Q, Afroz S, Golam M, Amarasiriwardena C, Bellinger DC, Christiani DC, Coull BA, Wright RO. The Joint Effect of Prenatal Exposure to Metal Mixtures on Neurodevelopmental Outcomes at 20–40 Months of Age: Evidence from Rural Bangladesh. Environ Health Perspect. 2017;125 doi: 10.1289/EHP614. https://doi.org/10.1289/EHP614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valvi D, Casas M, Romaguera D, Monfort N, Ventura R, Martinez D, Sunyer J, Vrijheid M. Prenatal Phthalate Exposure and Childhood Growth and Blood Pressure: Evidence from the Spanish INMA-Sabadell Birth Cohort Study. Environ Health Perspect. 2015;123 doi: 10.1289/ehp.1408887. https://doi.org/10.1289/ehp.1408887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valvi D, Mendez MA, Martinez D, Grimalt JO, Torrent M, Sunyer J, Vrijheid M. Prenatal Concentrations of Polychlorinated Biphenyls, DDE, and DDT and Overweight in Children: A Prospective Birth Cohort Study. Environ Health Perspect. 2011;120:451–457. doi: 10.1289/ehp.1103862. https://doi.org/10.1289/ehp.1103862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Berg H. Global Status of DDT and Its Alternatives for Use in Vector Control to Prevent Disease. Environ Health Perspect. 2009;117:1656–1663. doi: 10.1289/ehp.0900785. https://doi.org/10.1289/ehp.0900785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhulst SL, Nelen V, Hond ED, Koppen G, Beunckens C, Vael C, Schoeters G, Desager K. Intrauterine exposure to environmental pollutants and body mass index during the first 3 years of life. Environ Health Perspect. 2009;117:122–126. doi: 10.1289/ehp.0800003. https://doi.org/10.1289/ehp.0800003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner M, Aguilar Schall R, Harley KG, Bradman A, Barr D, Eskenazi B. In utero DDT and DDE exposure and obesity status of 7-year-old Mexican-American children in the CHAMACOS cohort. Environ Health Perspect. 2013;121:631–636. doi: 10.1289/ehp.1205656. https://doi.org/10.1289/ehp.1205656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner M, Wesselink A, Harley KG, Bradman A, Kogut K, Eskenazi B. Prenatal Exposure to Dichlorodiphenyltrichloroethane and Obesity at 9 Years of Age in the CHAMACOS Study Cohort. Am J Epidemiol. 2014;179:1312–1322. doi: 10.1093/aje/kwu046. https://doi.org/10.1093/aje/kwu046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitworth KW, Bornman RM, Archer JI, Kudumu MO, Travlos GS, Wilson RE, Longnecker MP. Predictors of Plasma DDT and DDE Concentrations among Women Exposed to Indoor Residual Spraying for Malaria Control in the South African Study of Women and Babies (SOWB) Environ Health Perspect. 2014 doi: 10.1289/ehp.1307025. https://doi.org/10.1289/ehp.1307025. [DOI] [PMC free article] [PubMed]
- WHO. WHO | Double burden of malnutrition [WWW Document] WHO; 2016a. URL http://www.who.int/nutrition/double-burden-malnutrition/en/ (accessed 2.8.17) [Google Scholar]
- WHO. Levels and trends in child malnutrition: UNICEF -WHO -The World Bank joint child malnutrition estimates. UNICEF; New York: WHO; Geneva: The World Bank; Washington, DC: 2016b. [Google Scholar]
- WHO. Levels and trends in child malnutrition: UNICEF -WHO -The World Bank joint child malnutrition estimates. UNICEF; New York: WHO; Geneva: The World Bank; Washington, DC: 2012. [Google Scholar]
- WHO. Child Growth Standards - WHO Anthro (version 3.2.2 January 2011) and macros. World Health Organization; Switzerland: 2011. [Google Scholar]
- Xu LC, Sun H, Chen JF, Bian Q, Song L, Wang XR. Androgen receptor activities of p,p′-DDE, fenvalerate and phoxim detected by androgen receptor reporter gene assay. Toxicol Lett. 2006;160:151–157. doi: 10.1016/j.toxlet.2005.06.016. https://doi.org/10.1016/j.toxlet.2005.06.016. [DOI] [PubMed] [Google Scholar]
- Yi N, Yandell BS, Churchill GA, Allison DB, Eisen EJ, Pomp D. Bayesian Model Selection for Genome-Wide Epistatic Quantitative Trait Loci Analysis. Genetics. 2005;170:1333–1344. doi: 10.1534/genetics.104.040386. https://doi.org/10.1534/genetics.104.040386. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


