Fig. 1.
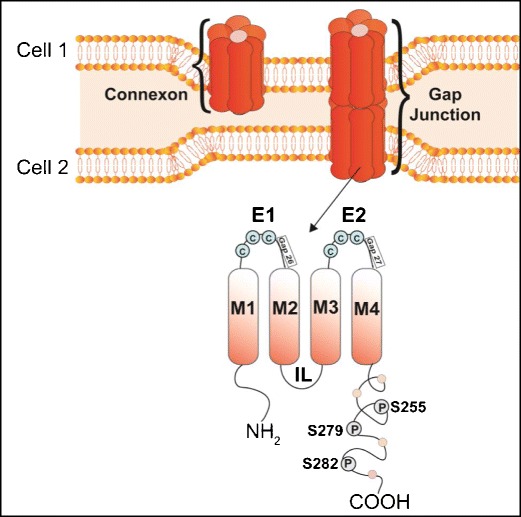
Structural organization of a connexin (Cx) protein, a connexon hemichannel, and a gap junction. One connexon or hemi-channel is formed by the association of six connexin (Cx) proteins. Two hexameric connexons between two adjoining cells dock to form a gap junction channel. Topologically, one Cx is composed of four transmembrane domains (M1 to M4), two extracellular loops (E1 and E2), one intracellular loop (IL), and intracellular amino (NH2) and carboxy (COOH)-termini. E1 and E2 have three cysteine residues (C) that form disulfide bonds with adjoining Cx proteins, allowing the docking of two connexons. The Cx-mimetic blocking peptides Gap26 and Gap27 specifically target E1 and E2, respectively. The C-terminal is subjected to a variety of post-translational modifications, including phosphorylation at the S255, S279, and S282 sites by the mitogen-activated protein kinases (MAPK)
