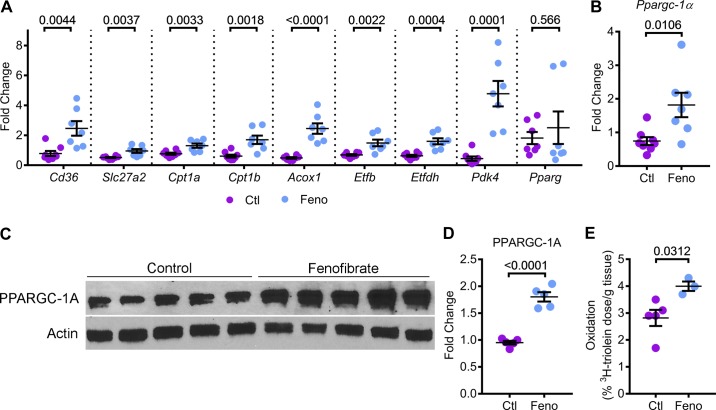
Fig. 3.
Fenofibrate enhances FAO and OXPHOS in Pkd1RC/RC mice. PPARα regulates several aspects of mitochondrial metabolism, including OXPHOS and FAO. Therefore, we tested whether fenofibrate treatment and PPARα upregulation affected kidney mitochondrial metabolism. A: Q-PCR analysis of FAO and OXPHOS genes that are directly regulated by PPARα was performed (n = 7 or 8). Expression of metabolism-related genes Cd36, Slc27a2, Cpt1a, Cpt1b, Acox1, Etfb, Etfdh, and Pdk4 was significantly increased in kidneys of fenofibrate-treated Pkd1RC/RC mice compared with control-treated Pkd1RC/RC mice. Fenofibrate treatment did not affect the expression of Pparg, which encodes PPARγ, a member of the PPAR subfamily. B–D: Q-PCR (n = 7 or 8) and Western blot analysis (n = 5) showed that the expression of Ppargc-1a mRNA and PPARGC1-α protein levels were increased in the kidneys of fenofibrate-treated Pkd1RC/RC mice compared to control-treated Pkd1RC/RC mice, suggesting that fenofibrate treatment was associated with enhanced mitochondrial biogenesis. E: to measure whether improved PPARα metabolic gene network was associated with improved mitochondrial function, control (n = 5) and fenofibrate-treated (n = 3) Pkd1RC/RC mice were injected with 3H-labeled triolein tracer to measure in vivo kidney FAO. Oxidation of 3H-labeled triolein was increased by 41.8% in fenofibrate-treated compared to control-treated Pkd1RC/RC mice. Ctl, control; feno, fenofibrate. Error bars represent means ± SE.