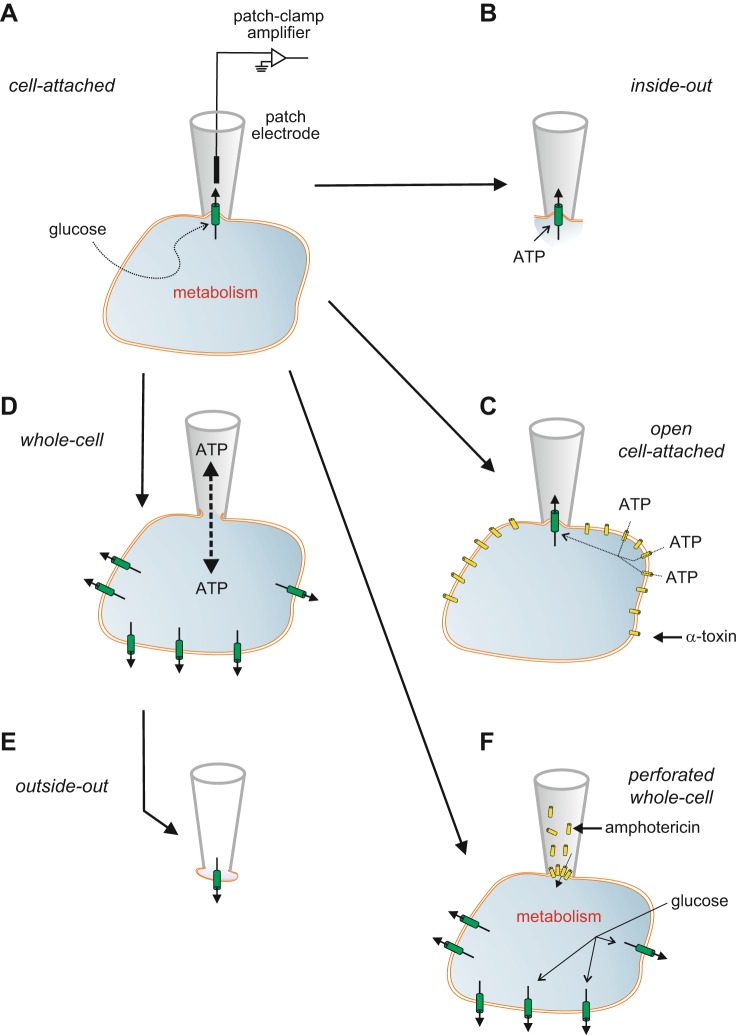
FIGURE 3.
Patch-clamp techniques. A: the experiments start with establishment of the cell-attached configuration. In this recording mode, a patch electrode is tightly sealed to the surface of an intact cell, allowing channel activity in the patch of membrane under the electrode tip to be studied under physiological conditions. For example, changes in channel activity in response to glucose metabolism can be measured by adding glucose to the bath solution. The seal between the electrode and the membrane is mechanically very stable, which enables additional configurations to be obtained. B: upon withdrawal of the electrode, the piece of membrane spanning the electrode tip is ripped off, forming an excised membrane patch that has its intracellular surface exposed to the bath solution (an inside-out patch). This is used for testing the effects of cytosolic constituents, such as ATP, on channel function. C: the plasma membrane outside the recording electrode can be permeabilized using detergents [like digitonin or saponin (162)] or the pore-forming peptide α-toxin [from Staphyloccus aureus (674)] to allow exchange of small molecules with a diameter of <1.5 nm (such as ATP) but not larger molecules (like enzymes). This recording configuration is referred to as the open cell-attached. D: the membrane beneath the electrode tip can be destroyed by suction, providing electrical access to the cell interior. This is known as the standard whole-cell configuration as it measures the summed activity of all ion channels in the cell membrane. It allows dialysis of the cell contents with the pipette solution. For example, the intracellular ion concentrations and cytosolic constituents (like ATP) can be manipulated by this route. The whole-cell configuration can also be used to preload the cells with biologically inert precursors of intracellular regulators that can then be photoliberated by a flash of ultraviolet light (‟caged compoundsˮ). E: withdrawal of the pipette from the standard whole-cell configuration produces an outside-out patch, in which the external membrane surface faces the bath solution. This is used to test the effects of extracellular ligands on channel activity. It can also be used as a ‟snifferˮ patch to probe the release of substances from the β-cell, if the membrane patch contains receptors to the compound of interest. F: the perforated patch whole-cell configuration allows measurement of electrical activity or whole-cell currents from a metabolically intact cell (291). In this variant of the whole-cell configuration, a pore-forming antibiotic [such as amphotericin (531)] is incorporated into the membrane below the pipette tip, thereby establishing electrical access to the cell while leaving cellular metabolism and intracellular second messenger systems intact.