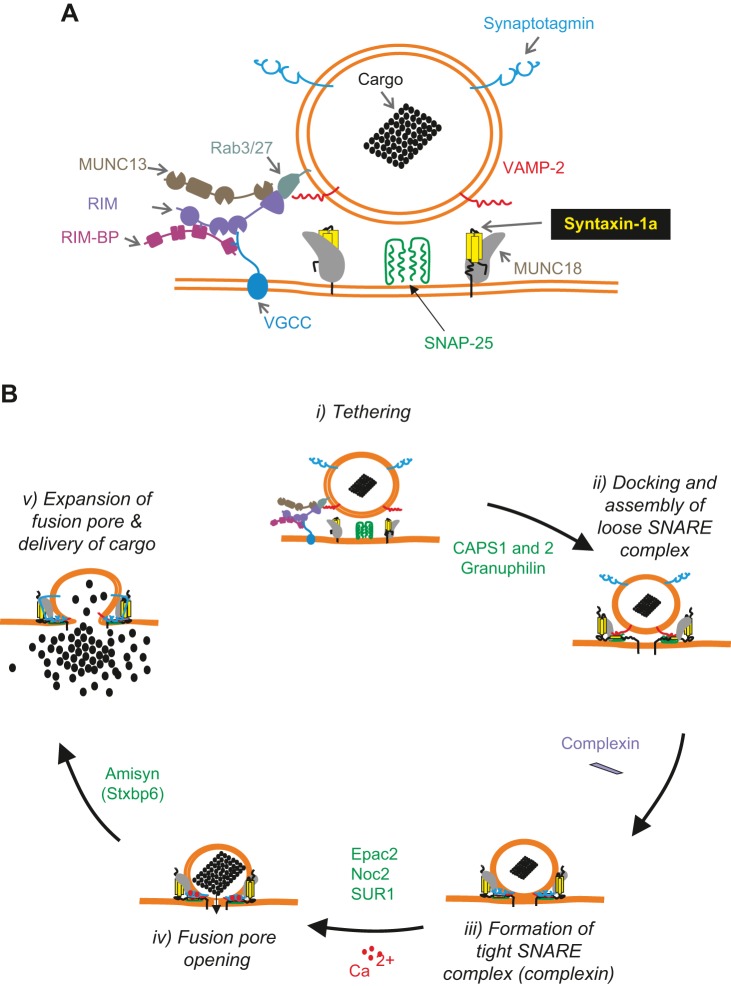
FIGURE 16.
Schematic of the molecular machinery mediating Ca2+-dependent synaptic vesicle release. A: the core fusion machinery comprises the SNARE/SM protein complex. It consists of the vesicular (v-)SNARE protein VAMP-2 (red), the plasma membrane (target, t) SNARE proteins syntaxin-1 (yellow), and SNAP-25 (green), Munc18–1 (gray), and the Ca2+-sensor synaptotagmin-1 (blue, on vesicle). Tethering of the synaptic vesicle to the active zone involves a plasmalemmal voltage-gated Ca2+ channel (VGCC) and an active zone protein complex consisting of RIM (violet), Munc13 (brown), and RIM-BP (purple). RIM binds to the vesicular rab proteins Rab3 and Rab27 (sea green). B: five stages of exocytosis are illustrated: i) tethering; ii) docking and assembly of a loose trans-SNARE complex; iii) the formation of a tight 4-helix or ternary SNARE complex, with one helix coming from syntaxin, two helices from SNAP-25 and one helix from VAMP-2. This process is stabilized by complexin; iv) Ca2+ binding to synaptotagmins results in displacement of complexin, completion of zippering, and fusion pore opening; and v) expansion of the fusion pore and release of the cargo (a dense core vesicle is shown rather than a clear synaptic vesicle). After fusion, the resulting cis-SNARE complexes (cis, in the same membrane; trans, in opposite membrane) are disassembled by the NSF/SNAP ATPases and recycled. Modulators of β-cell exocytosis and where they act are given in green (see sect. IX, A and B). [Modified from Südhof (652).]