FIGURE 17.
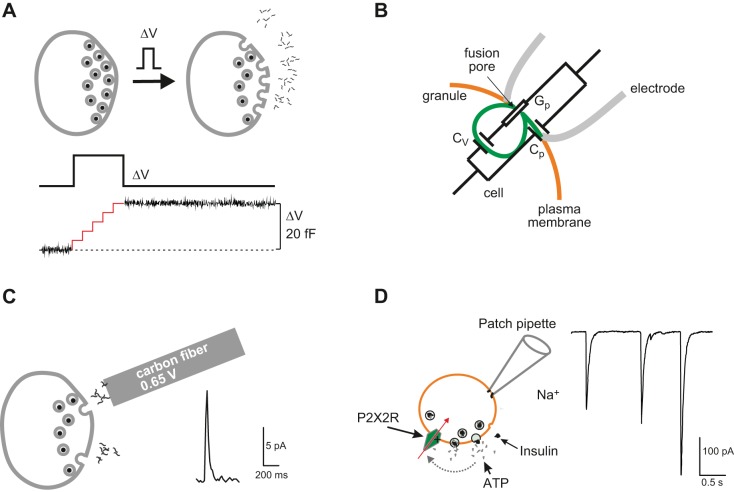
A: capacitance measurements of exocytosis. Ca2+ influx triggered by a brief depolarization (ΔV) leads to the fusion of (five) secretory granules with the plasma membrane (gray). The resultant increase in membrane area can be detected as an increase in cell capacitance (ΔC) because cell capacitance (C) is proportionally related to cell surface area (A) [i.e., C = ε*A, where ε is the specific membrane capacitance (10 fF/μm2)]. For technical reasons, the recording is usually interrupted during the depolarization (illustrated schematically by the red trace). The net increase in cell capacitance (ΔC) that occurred during the pulse is shown by the black trace (334). B: schematic of on-cell (cell-attached) single-granule capacitance measurements and the equivalent circuit. Orange, green, and gray lines correspond to the plasma membrane, the granule membrane, and the walls of the recording pipette, respectively (not to scale). Gp, Cv, and Cp are fusion pore conductance, granule capacitance, and patch capacitance, respectively. [Modified from Lindau (397).] C: carbon fiber amperometry. A carbon fiber connected to an amplifier is placed in the vicinity of the cell. Exocytosis can be detected as amperometric current spikes (right) that develop when the substance released (e.g., serotonin) is oxidized by the high voltage (e.g., 0.65 V) applied to the carbon fiber giving rise to a rapid current transient (right). D: electrophysiological detection of ATP release. ATP is co-released with insulin and activates P2X2 receptors (P2X2Rs) in β-cells engineered to express such receptors. ATP release and activation of P2X2Rs result in rapid current transients.