FIGURE 20.
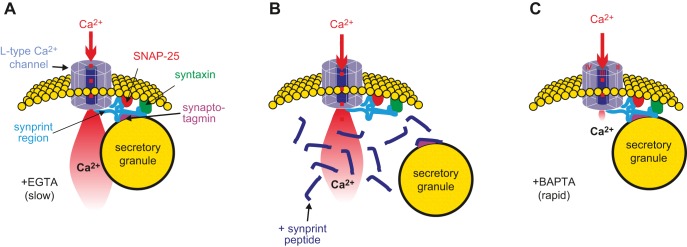
Tethering of voltage-gated Ca2+ channels to secretory granules. A: SNAREs bind to the II-III loop of the L-type Ca2+ channel, the synaptic protein interaction (synprint peptide), and thereby tether the granule close to the inner mouth of the channel. Upon Ca2+ channel activation, the exocytotic machinery becomes exposed to a localized increase in [Ca2+]i. These transients are too rapid to be buffered by slow Ca2+ chelators like EGTA, explaining why depolarization-evoked exocytosis is resistant to this Ca2+ buffer. B: following the addition of a large excess of the ‟synprint peptide,ˮ the endogenous synprint peptide (which is part of the Ca2+ channel) is competitively displaced, leading to the disassembly of the granule-channel complex. Although Ca2+ channel activity is unperturbed, the secretory granule is no longer sufficiently close to the inner mouth of the Ca2+ channel (where [Ca2+]i exists at exocytotic levels), leading to the suppression of insulin release. C: the rapid Ca2+ chelator BAPTA binds Ca2+ so quickly that even granules tethered to the inner mouth of the Ca2+ channels are not exposed to Ca2+ concentrations high enough to evoke secretion (right). Figure courtesy of Professor E. Renström, Lund.