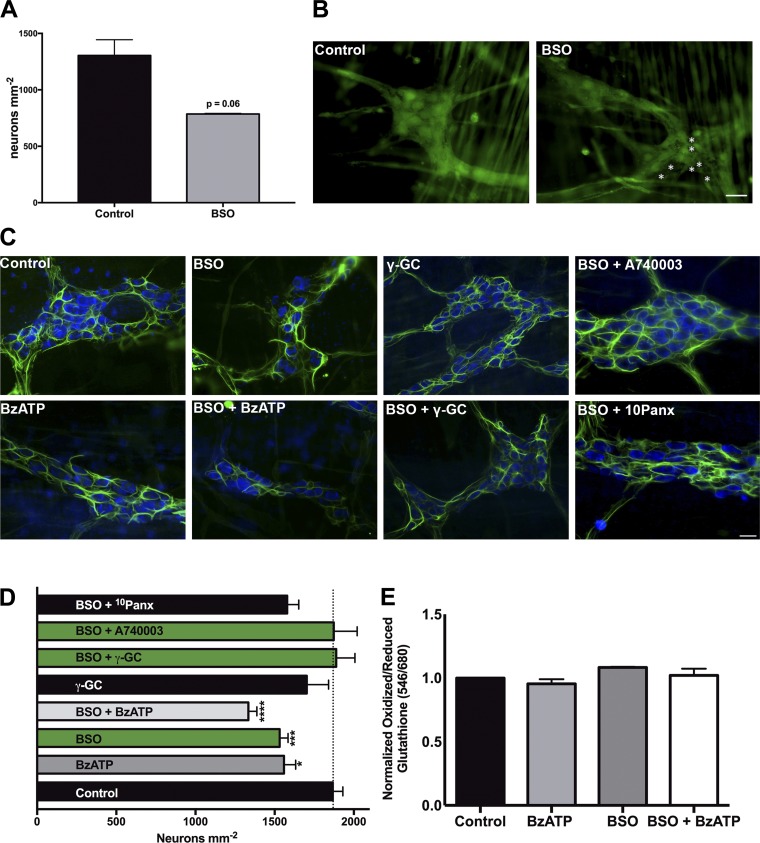
Fig. 4.
In situ inhibition of glutathione (GSH) synthesis contributes to neuron death in a P2X purinoceptor 7 receptor (P2X7R)-dependent manner. Quantification (A) and representative images (B) of live enteric neurons in myenteric ganglia following in situ depletion of GSH with l-buthionine-sulfoximine (BSO, 100 μM) or buffer control treatment. Live cells are labeled with the fluorescent marker calcein AM (green), and dead neurons are denoted by white asterisks. Representative images (C) and quantification (D) of mean neuronal packing density in mouse myenteric ganglia following inhibition of GSH synthesis with the glutamate-cysteine ligase catalytic subunit (GCLC) inhibitor BSO (100 μM). Whole mount preparations of myenteric plexus were incubated with BSO in the presence, or absence, of the GCLC product γ-glutamyl-cysteine (γ-GC, 100 μM), the P2X7R agonist 2′(3′)-O-(4-benzoylbenzoyl)adenosine 5′-triphosphate triethylammonium salt (BzATP, 300 μM), the P2X7R antagonist A740003 (10 μM) or the pannexin mimetic peptide 10Panx (100 μM). Inhibition of BSO decreases neuronal density to the same extent as P2X7R activation, while simultaneous treatment with γ-GC, or inhibition of P2X7R, protects against BSO-mediated cell death. (*P ≤ 0.05, ***P ≤ 0.0005, ****P ≤ 0.0001, 1-way ANOVA with Dunnett’s multiple-comparison test vs. control, n = 3–19 myenteric preparations per group from 15 animals.) Enteric neurons are labeled with HuC/D (blue), and enteric glia are labeled with glial fibrillary acidic protein (GFAP, green) in all panels. E: neuronal thiol oxidation measurements (ratio of fluorescently labeled oxidized glutathione to reduced glutathione) from in situ myenteric preparations treated with BSO (100 μM) in the presence or absence of BzATP (300 μm) (n = 3–6 ganglia per group). (Scale bar = 30 μm in all images.)