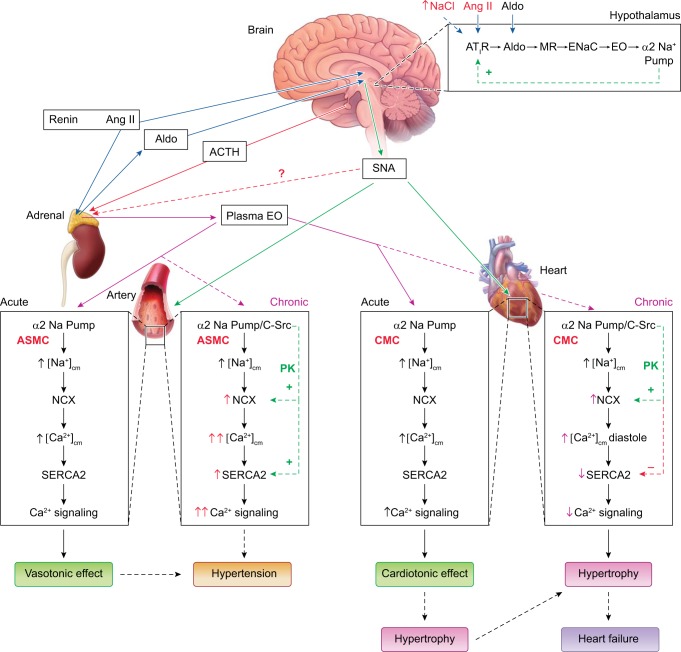
Fig. 4.
The centrally controlled, parallel sympathetic nerve and slow neurohumoral pathways that regulate both arterial and cardiac function, and participate in the pathogenesis of hypertension and heart failure (HF). Angiotensin II (Ang II) and high dietary salt (↑[Na+]) are convergent signals that act via hypothalamic Ang-type 1 receptors (AT1R) to activate central nervous system (CNS) sympathetic pathways; the role of the kidneys in the retention of salt has been omitted for the sake of simplicity. The increased sympathetic nerve activity (SNA) promotes vasoconstriction and increases cardiac rate and contractile force. Prolonged stimulation of hypothalamic AT1R also activates a novel neurohumoral pathway (box at top right) that includes aldosterone (Aldo), mineralocorticoid receptors (MR), epithelial Na+ channels (ENaC), endogenous ouabain (EO), and α2 Na+ pumps. This hypothalamic pathway feeds back (green line, “+”) to modulate Ang II-activated SNA and also promotes adrenal secretion of EO, triggered by, e.g., ACTH, adrenal SNA, and/or Ang II. The elevated plasma EO acutely inhibits α2 Na+ pumps in both the heart and arteries, and the rise in intracellular Na+ rapidly induces Na/Ca exchanger (NCX)-mediated Ca2+ gain, and cardiotonic and vasotonic effects. Prolonged plasma EO elevation also activates an α2 Na+ pump-associated protein kinase cascade (e.g., EGFR/Src/MEK/ERK-mediated) that increases cardiac and arterial NCX expression, and arterial sarcoplasmic reticulum (SR) Ca2+ pump (SERCA2) expression. In arteries with tone, NCX normally favors Ca2+ entry and helps to sustain cytosolic Ca2+ ([Ca2+]CYT) above contraction threshold. The EO-induced NCX and SERCA2 upregulation enhances Ca2+ signaling and helps the very modestly increased SNA to increase vascular tone and resistance and elevate blood pressure. In the heart, NCX promotes Ca2+ extrusion during diastole, but prolonged α2 pump inhibition by EO reduces the Na+ gradient driving force so that [Na+]CYT and diastolic [Ca2+]CYT are both elevated; consequently, cardiac relaxation is slow and/or incomplete. Also, cardiac SERCA2 expression is usually reduced in HF (perhaps due to the high EO), as are SR Ca2+ stores and [Ca2+]CYT transients, and systolic function is impaired. The diastolic dysfunction and attenuated cardiac contraction and stroke volume contribute to HF. These cellular mechanisms are discussed further in a recent review (31). [Modified from Blaustein et al. (31) with permission.]