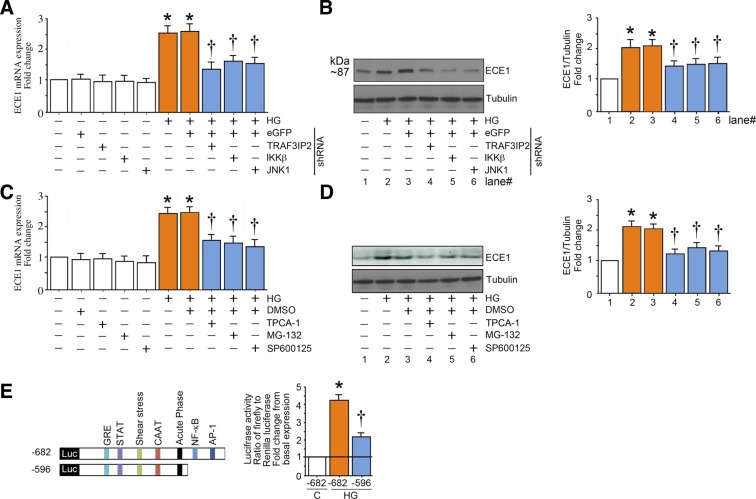
Fig. 3.
High glucose (HG) induces endothelin converting enzyme 1 (ECE1) expression in part via TRAF3 interacting protein 2 (TRAF3IP2) and its downstream signaling intermediates. A and B: HG (25 mM) induces ECE1 mRNA and protein expression in part via TRAF3IP2, IKKβ, and JNK. At 50% confluency, human aortic endothelial cells (HAECs) infected with lentiviral TRAF3IP2, IKKβ, JNK1, or control eGFP shRNA [multiplicity of infection (MOI) 0.5] for 48 h were treated with HG for 120 min. ECE1 mRNA expression (A) was analyzed by quantitative RT-PCR and protein levels (B) by immunoblot analysis. C and D: pharmacological inhibition of IKKβ, NF-κB, and JNK inhibit HG (25 mM)-induced ECE1 expression. At 70% confluency, the complete medium on HAECs was replaced with endothelial basal medium-2 (without supplements) for 2 h, pretreated with TPCA-1 (5 µM in DMSO), MG-132 (5 µM in DMSO for 1h), SP600125 (20 µM for 30 min), or DMSO vehicle, and then incubated with HG and analyzed for ECE1 mRNA (C) and protein expression (D) after 2 h as in A. E: HG (25 mM) stimulates ECE1 promoter-dependent reporter gene activation via NF-κB and AP-1. HAECs were transfected with a reporter vector containing a 682-bp fragment of the 5′-flanking region of the human ECE1 gene (3 μg for 24 h) with and without the deletions. pGL3-Basic served as a vector control. Cells were cotransfected with the Renilla luciferase vector (100 ng). After transfection, cells were treated with HG for 12 h and harvested for the dual-luciferase assay (n = 6). Firefly luciferase data were normalized to that of corresponding Renilla luciferase activity. Bar graphs in A–D represent densitometric analyses from at least 3 independent experiments. A–E: *P ≤ 0.01 vs. control (i.e., first open bar); †P ≤ 0.05 vs. HG (n = 3–6).