Summary
Collapse of the femoral head is the most significant pathogenic complication arising from osteonecrosis of the femoral head. It is related to the disruption of the maintenance of cartilage and bone, and results in an impaired function of the vascular component. A method for predicting the collapse of the femoral head can be treated as a type of clinical index. Efforts in recent years to predict the collapse of the femoral head due to osteonecrosis include multiple methods of radiographic analysis, stress distribution analysis, finite element analysis, and other innovative methods. Prediction methods for osteonecrosis of the femoral head complications originated in Western countries and have been further developed in Asia. Presently, an increasing number of surgeons have chosen to focus on surgical treatments instead of prediction methods to guide more conservative interventions, resulting in a growing reliance on the more prevalent and highly effective total hip arthroplasty, rather than on more conservative treatments. In this review, we performed a literature search of PubMed and Embase using search terms including “osteonecrosis of femoral head,” “prediction,” “collapse,” “finite element,” “radiographic images,” and “stress analysis,” exploring the basic prediction method and prospects for new applications.
Keywords: clinic application, collapse, femoral head, finite element, osteonecrosis, radiographic analysis
Introduction
Osteonecrosis of the femoral head (ONFH) is a progressive process due to multiple factors affecting the blood supply of the femoral head and the disruption of the synthesis of the bone component [1]. Collapse of the femoral head is one of the most severe complications; it may cause intolerable symptoms such as hip pain, dysfunction, and claudication, and seriously impact the quality of life of patients [2], [3]. Despite the unclear mechanisms contributing to a collapse, factors affecting the bone necrotic area were found to be related to significant biomechanical changes in the load-bearing area of the femoral head, including microfractures, collapses, bone deformation, bone degeneration, etc. [4].
Collapse prediction was proposed for the first time in 1974 by Kerboul et al [5], with the growing prevalence of X-rays. Over the past 50 years, many surgeons have gradually come to consider a collapse due to ONFH not only as a critical point for femoral head survival, but also as the evaluation standard for early treatment. In Asian countries, there have been many studies on the mechanism of the femoral head collapse and the prediction and prevention of a collapse [6]. Despite the limitations of radiographic examination, X-rays and magnetic resonance imaging (MRI), in particular, have had great value in predicting a collapse in ONFH, although some other factors, such as necrotic features, phases, or aetiology, are also involved [7]. Previous studies have generally been limited to a certain geographic region. Meanwhile, sample size has also been limited by the researchers [8]. However, the factors mentioned above are mutually interactive with each other and appear to a play role together in the development of a collapse. There is a lack of a consensus agreement on the different methods for preventing a collapse in ONFH. Therefore, a comprehensive review is necessary.
Biological mechanisms
Physical stress is one of the most important causes of ONFH [1], [9]. The bone repair process occurring along with ONFH is constantly involved with some level of reconstruction and remodelling of bone tissue. These reconstruction processes result from the adaptation of bone tissues to an external load and have been demonstrated in animal experiments [10]. The rate of deformation developed in necrotic cartilage and bone is lower, which leads to an uneven mechanical transmission from the joint surface to the trabecular bone. An abnormal increase of hip joint stress occurs when the compliance of the cartilage and adjacent bone decreases. Finally, the stress concentrates along the interface of the necrotic bone and the normal bone, and continues to produce sclerotic band formation and microfractures of the bone, even after the collapse of the femoral head [11].
Biomechanical principle
In the initial stages of a collapse, proliferation of osteoblasts and activity of osteoclasts increase at the same time, resulting in net bone resorption [12]. Later, when the necrotic areas are in a pathological state of low nutrition and low oxygen, proliferation of osteoblasts is inhibited [13], alkaline phosphatase activity is decreased, and osteoclasts, fibroblasts, fat cells, and chondrocytes are stimulated to proliferate [14]. Osteoblast and osteoclast coupling is imbalanced, and osteogenesis is defective, resulting in the failure of the repair process [15]. Necrotic bone is not well reconstructed during the process of absorption and creeping substitution [16].
Location and range
Location of necrosis was closely related to the occurrence and site of the collapse [17]. Typically, collapses do not occur in cartilaginous regions, but usually occur in the necrotic bone or the newly formed bone, even though the cartilage is the first direct area to be subjected to stress. When the mechanical properties of incompletely calcified bone or new bone in a particular area cannot withstand the stress, a collapse is likely to occur. The occurrence of a collapse can be related to structural differences in different necrotic areas, including medial, lateral, and front regions [18]. Considering the necrotic range, there is a certain relationship between the size of the necrotic area and the probability of a collapse [19]. Specific areas of necrosis can directly affect the survival ability of the femoral head. For example, if the necrotic area is small or distal to the cartilage bone, it may repair and heal.
Phases
The occurrence of a collapse is a direct result of the combined effects of the biological and biomechanical properties of the necrotic femoral head during the repair phase. Biological responses lead to a significant decrease in biomechanical durability, especially of the cartilage, which may be the major factor leading to a collapse. Meanwhile, a collapse occurs during the repair process, with multiple related factors including location, scope, aetiology, etc. Note that the collapse occurs during the repair phase, rather than in the early stages of necrosis. There are many methods to classifying the stages of ONFH, such as the ARCO phase, Ficat classification [20], [21]. Judging the extent of femoral head osteonecrosis is a classification method commonly used for the prediction of a collapse. Details on these published methods are noted in this review (Figure 1).
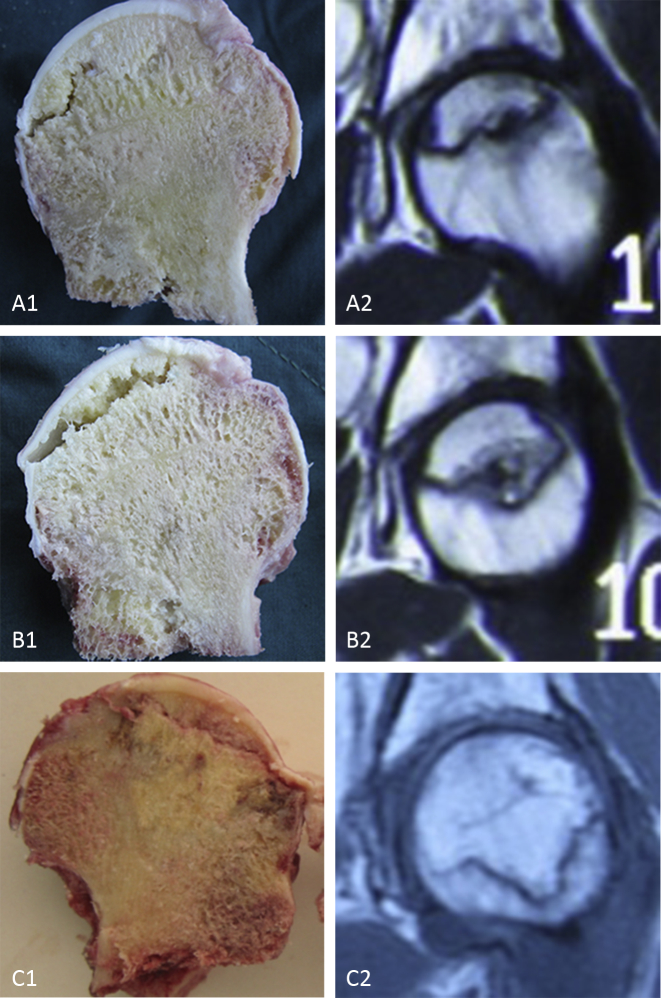
Figure 1.
Gross specimens of femoral head osteonecrosis and the relative MR images. (A1, A2) ONFH in ARCO IIIA phase, (B1, B2) ONFH in ARCO IIIB phase, and (C1, C2) ONFH in ARCO IIIC phase. Collapse of the femoral head, the most significant characteristic, is usually considered to occur in ARCO III phase. Effective conservative measures are required to be taken before such phase, while some surgeons also insisted that good results can be obtained even after the occurrence of a collapse. ARCO = Association Research Circulation Osseous; MR = magnetic resonance; ONFH = osteonecrosis of the femoral head.
Methods
Data source
A computer-based retrieval was performed by the first author in PubMed (http://www.ncbi.nlm.nih.gov/pubmed/), Google (http://www.scholar.google.com.hk) and SpringerLink (http://springer.lib.tsinghua.edu.cn/) databases for literatures published from November 1970 to November 2015, using the keywords “osteonecrosis of femoral head,” “prediction,” “collapse,” “finite element,” “radiographic images,” and “stress analysis.”
Data selection
Papers meeting the following criteria were included: paper written in English or Chinese; papers with contents closely related to this paper; original papers with reliable topics and evidence; and papers with clear points and all-round analysis. Obsolete and repetitive studies were excluded.
Percentage of necrotic area analysis
The necrotic area percentage method is used to estimate the probability of a collapse at the initial examination because a higher percentage indicates that the bone structure is more seriously affected, after discounting other factors. To some extent, the necrotic area reflects the severity of necrosis and the mechanical interstructure. The primary analysis was simply based on a necrosis size determination. Sugano et al [22] began to develop an outline of the necrotic and normal areas with a calculation, using the following formula: necrotic area percentage = SN/(SN + SI) × 100%. Their results showed that the necrotic area percentages in the lateral collapse group were significantly greater than those in the noncollapse group. When the percentage of the necrotic area in the anteroposterior and lateral radiographic positions was 30% or less, no collapse was observed. This method creatively used several one-dimensional images to reflect the three-dimensional space. Koo et al [23] determined the femoral head necrotic angle based on the abnormal signal in the middle coronal and sagittal images of T1-weighted MRI. They established a point on the outside edge as A and an inner edge point as B. Then, a necrosis index was determined using the following formula: necrosis index = (A/180) × (B/180) × 100%. According to this index, a lesion with a calculated necrosis index was assigned to one of the following three size levels: class A for <33%; class B for 33–66% or moderate necrosis; and class C for 67–100%, with an extensive necrotic area. According to the authors, if this index is higher than 40%, the femoral head is considered prone to a collapse. Weight-bearing factors were later integrated into the index calculation [24]. The updated index formula used was as follows: weight bearing = β/γ × 100% (β is the range of necrotic area in the weight-bearing area and γ the range of weight-bearing area). No imminent collapse was found when the weight-bearing index was less than 45% (Table 1).
Table 1.
Percentage of necrotic area analysis in classification and correlation with collapse.
| Author | Year | Country | Study point | Classification | Relations with collapse |
|---|---|---|---|---|---|
| Kerboul et al [5] | 1974 | France | Necrosis radian | Large range: ≥200 Small range: ≤160 Median range: >160, <200 |
Large range: worse result Small range: better result |
| Beltran et al [25] | 1990 | United States | Percentage of necrotic area | — | No collapse occurs in the range <25%, 43% collapse in the range from 25% to 50%, and 87% collapse in the range of 50%. |
| Sugano et al [22] | 1994 | Japan | Necrosis portion and range (X-ray) | IA < 30% IB < 44% IC < 88% |
— |
| Koo and Kim [26] | 1994 | South Korea | Necrosis range | Divided into three types: <30%, 30–40%, >40% | Collapse rate increases in the upper type. |
| Koo et al [23] | 1995 | South Korea | Necrosis index (necrosis range) | Necrosis index = (A/180)(B/180)(C/180) A, B, C are three angles |
A level: <33 B level: 33–60 C level: 67–100 If the index is >40, collapse of the femoral head occurs. |
| Steinberg et al [27] | 1999 | United States | Necrosis radian | — | Necrosis radian >200, worse result |
| Shimizu et al [28] | 1994 | Japan | Necrosis range | Grade: high/low sign Grade: high sign |
Grade is confer to be collapse. Grade in the critical range, like weight-bearing area, is vice versa. |
| Chen et al [29] | 2000 | China | Necrosis radian | — | In core depression operation, all the cases whose necrosis radian is >250 collapse. |
| Lafforgue et al [24] | 1993 | France | WB value (percentage of necrotic area vs. weight-bearing area) | WB = β/γ × 100% β: range of necrotic area in weight-bearing area γ: range of weight-bearing area |
No collapse when WB is <45%, but collapse occurs when WB is >45%. |
| Zhao et al [30] | 2005 | China | Necrosis index Percentage of necrotic area |
Some kind of index for predicting necrosis | The relative risk of the percentage of necrotic surface area is 1.043. |
| Ha et al [31] | 2006 | South Korea | Percentage of necrotic area | Modified Kerboul et al’s [5] method: Level 1: <200; Level 2: 200–250; Level 3: 250–300; Level 4: >300 | — |
| Connolly and Weinstein [32] | 2006 | Turkish | Necrosis range | — | Necrotic area <33% would not favour a collapse. |
Location analysis of necrosis
Location analysis of necrosis is extremely common in clinical practice and is closely linked with the ONFH phase for collapse prediction. As originally used in X-ray images, location analysis was then introduced to MRI, which provided orientation for a series of core decompression surgeries, due to the emergence of its accurate location. Ohzono et al [17] made their first attempt and created one kind of classic methods in 1991 and 1992. Their teams divided the location of necrotic area into three types based on the X-ray image for confirming the site, from sclerosis rim, weight-bearing necrotic area, to cystic degeneration, and so on. The first subtype was further divided into Grade A (less than 1/3 of the weight-bearing area), Grade B (about 1/3), and Grade C (outer side of 1/3). This method is more similar to clinical classification even though it has its limited value. On the basis of the former, Sugano et al [33] put forward their method to be applied to MRI. Based on the necrotic area in the middle coronal T1-weighted display, femoral head osteonecrosis was divided into three types. There is little difference from Ohzono et al’s [17] results. A type is within one-third of the femoral head, B type is no more than two-thirds of the weight-bearing area, and C type is larger than two-thirds of the weight-bearing area. The results showed that the incidence rate is high when C type is more than 50% in both coronal and sagittal images (Table 2).
Table 2.
Location analysis of necrosis in classification and correlation with collapse.
| Author | Year | Country | Study point | Classification | Relations with collapse |
|---|---|---|---|---|---|
| Ohzono et al [17] | 1991 1992 |
Japan | Osteonecrosis features (X-ray) | Type I: sclerosis band formation (IA, IB, IC) Type II: weight-bearing area deformation Type III: cystic change |
IC, IIB, IIIB are more likely to collapse. |
| Sugano et al [33] | 1994 | Japan | Necrosis portion and range (MRI) | A type: <1/3 of the weight-bearing area; B type: <2/3; C type: >2/3 | C type, with above 50% of necrotic area in both the coronal and the sagittal position, is more likely to collapse. |
| Takatori et al [34] | 1996 | Japan | Necrosis portion | A type: anterior medial of the head C type: half of the head A type < B type < C type D type > C type |
Collapse rate increases in the upper type. Collapse is correlated with necrosis portion and range. |
| Sakamoto et al [35] | 1997 | Japan | Necrosis portion and range | Add a D type based on Sugano et al’s [33] study: C type does not expand outside the acetabular, but D type does. | D type seldom leads to a collapse. |
| Ito et al [36] | 1999 | Japan | Necrosis portion and range | Sakamoto et al’s [35] | Relatively stable microstructure of the femoral head can maintain an asymptomatic stage. |
MRI signal analysis
Hip MRI signal strength reflects a variety of pathological changes in the femoral head. For example, T1 sequences appear as high signals indicating the presence of oedema [37]. A high signal in ONFH generally demonstrates that violent changes have taken place in the bone structure. Collapses, pathological fractures, and other deteriorated structures produced relative signals such as double-line signs [38]. Kokubo et al [39] thought that MRI signals could be divided into five types: type A represents a wide range of low signals, type B low signals at the anterior–lateral position, type C a transverse low-signal band, type D scattered uneven low-signal spots within the head, and type E low signals in the distal femoral head. Their results suggest that the sclerotic rim, the low sign, across the femoral head would more likely aggravate the collapse in the coronal images of MRI. Takatori et al [40] divided the signals into four types according to the location and extent of fat intensity. Fat intensity limited to the medial–anterior femoral head was A-type; occupied a whole head for C-type; situated between A and C for B; D is larger than C. The authors believed that location and extent of fat intensity reflected the necrotic range and were closely related to the collapse. Koo et al [41], in their research on patients suffering from early-stage ONFH, found that bone marrow oedema around the necrotic area has a close relationship with hip pain. An oedema signal is considered by the authors to reflect the collapse (Table 3).
Table 3.
MRI signal analysis of necrosis in classification and correlation with collapse.
| Author | Year | Country | Study point | Relations with collapse |
|---|---|---|---|---|
| Bassett et al [42] | 1987 | United States | Lower stratum of diminished signal | The next lower stratum of diminished signal intensity was composed of fibrous and vascular tissues, and histiocytic infiltrates that had extensively or completely replaced the fatty marrow. |
| Wang et al [43] | 1998 | China | Trabecula in lower-signal area | Trabecula dispersion is less; mechanical strength is lower, which is preferred to a microfracture and collapse. |
| Iwasada et al [44] | 1999 | Japan | Low-intensity area | A low-intensity area or a low-intensity band in the new weight-bearing area extending over the acetabular edge on T1-weighted images was related to the presence of collapse. |
| Nishii et al [45] | 2002 | Japan | Lesion volume | Lesion volume and location (latitude and longitude). Lesion volume is closely related to collapse. |
MRI = magnetic resonance imaging.
Dimensional finite element analysis for necrotic volume
Volume analysis was achieved in a three-dimensional perception forecast using only the finite element technique. This method combined the percentage and location analyses though finite elements, which successfully constructed an ONFH structural model. Although the process of analysis remained complex and bloated, the method provided possible technical innovation in collapse prediction. Zhao et al [46] reconstructed a three-dimensional MRI image of the femoral head by finite element analysis. When the necrotic lesion volume was more than 30%, the collapse rate of the femoral head was as high as 80%; if the volume was less than 30%, while necrotic areas occupied the anterolateral part of the femoral head, collapse referred to happen. In addition, a necrotic volume of above 40% was likely to induce a collapse even under the normal load; however, a necrotic volume of <40% can also induce a collapse under increasing load.
Bone microarchitecture analysis
Kang et al [47] analysed the correlation between elastic modulus of cancellous bone, bone mineral density, and trabecular bone morphology and structure in 28 cases receiving total hip arthroplasty. They found that bone density can better reflect the biomechanical properties of the femoral head and microarchitecture of cancellous bone, which can be applied to predict the collapse of ONFH. Bone microstructure analysis was unable to detect a collapse in vivo and is still in its experimental stages [48]. The method, however, allowed us to clarify the mechanisms of the collapse in microscopic structures, providing appropriate clinical guidelines. Bone microarchitecture analysis may have considerable applications in the future [49]. For now, it has a high reference value for quantifying some key indicators of bone.
Bone scintigraphy, computed tomography, and emission computed tomography
Bone scintigraphy, computed tomography, and emission computed tomography were used to predict a collapse in ONFH based on the local blood flow and the level of bone metabolic activity [50], [51]. Using bone scintigraphy for predicting the collapse of the femoral head after femoral neck fracture, it was found that the dynamic changes in the image can be more accurate for understanding the femoral head in histologic restoration and partial metabolic process [52]. Sun et al [53] found that bone scintigraphy diagnosed in ONFH, compared with the X-ray, computed tomography, are found to be 3–6 months advance; 2–3 weeks advance compared with MRI. Additionally, sensitivity of bone scintigraphy is similar to MRI, but its specificity is low.
Stress distribution of necrotic area
Stress distribution of the necrotic area is a type of finite element analysis, which is based on the development in radiography and takes into account stress distribution analyses [54], [55]. Using this method, researchers will simulate the characteristics of various tissues around or in the femoral head by a finite element program, add increasing load gradually on the femoral head, and observe the collapse in the necrotic environment [56], [57] (Figure 2).
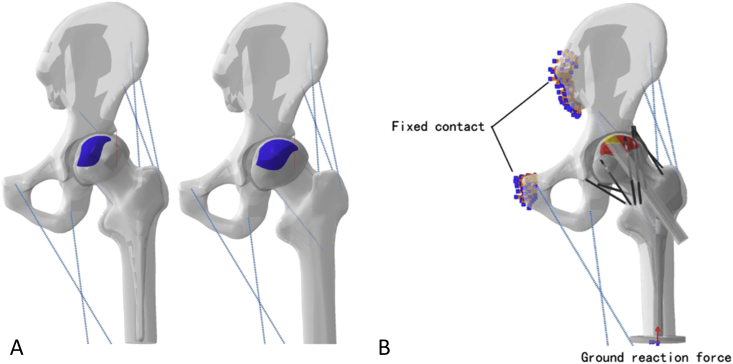
Figure 2.
(A) Three-dimensional subtype models of ONFH. (B) Load and constraint condition in the model of grafting treatment. ONFH = osteonecrosis of the femoral head.
Cui et al [58] established the femoral dimensional finite element model of the foreign bodies isomorphic. Simulation of the femoral head with cystic lesions (applying a variety of different loads) analysis of the stress distribution within the femoral head. The results suggest that stress obviously concentrated below the cystic lesions in the medial and lateral parts of the femoral head, easily causing the collapse of the femoral head. The authors mentioned that the biomechanical effects of various cystic lesions in different parts of the femoral head were different. Fang et al [59] set up the necrotic tissues with different volume fractions in an ONFH finite element model and added increasing load gradually. It was found that the range of necrotic area correlated with the stress distribution of the femoral head. That is, the stress is higher at the surface of the necrotic area than at the bottom; when the peak stress exceeds a critical value, a subchondral cancellous bone microfracture occurs. According to the authors, this should be the direct cause of the collapse (Figure 3).
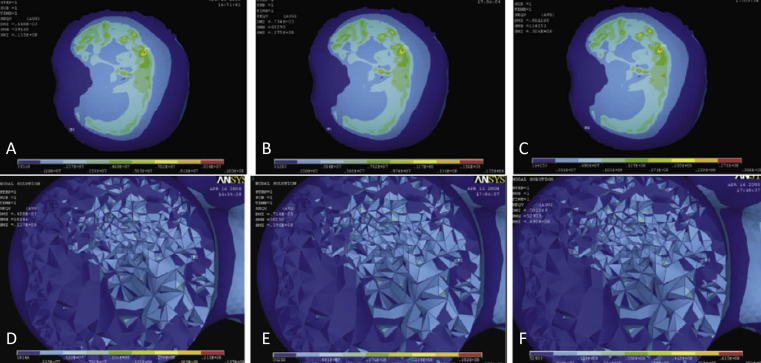
Figure 3.
Different loadings on the surface and bottom of the femoral head (60% necrosis). Surface group: (A) 1440 N loading, (B) 2400 N loading, and (C) 4200 N loading. Bottom group: (D) 1440 N loading, (E) 2400 N loading, and (F) 4200 N loading.
Analysis using 18F-fluoride positron emission tomography
It was reported that 18F-fluoride positron emission tomography was able to predict a collapse by scanning femoral head necrosis and get a maximum standardized uptake value (SUVmax) [60]. Kobayashi et al [61] first reported the positive relationship between SUVmax and the early stage of osteoarthritis. Dasa et al [62] previously mentioned that different expressions in the acetabular side on 18F-fluoride positron emission tomography indicated earlier acetabular metabolic changes in osteonecrosis. Kubota et al [63] found that the SUVmax value increases along with the increasing severity of osteonecrosis. Besides, they also focused on SUVmax to predict the collapse of ONFH patients in early phase. SUVmax of the collapse group was significantly higher than that of the noncollapse group, as revealed by a receiver operating characteristic curve. The critical threshold value of the SUVmax is 6.45, and the femoral head intends to collapse when the value is higher than 6.45. In addition, 18F-fluoride positron emission tomography was used in in vivo assessment of regional blood supply of the femoral head [64]. Long-time follow-up demonstrated its value for monitoring a collapse and prediction of outcome. Meanwhile, patients with severe hip pain had a significantly higher uptake value.
Cortical layer supporting
Finite element analysis showed that cancellous bone played a role in supporting the cortical bone and preventing the latter one from flexing. In case of the femoral head felled into necrosis, necrotic cancellous bone gradually lost its supporting effect, the threshold of pressure in femoral head. And lose cancellous bone supporting role, localized cortical bone will bend, deform deteriorate further. Volokh et al [65] found that this phenomenon will lead to a lower femoral critical load threshold. Curved femoral cortex may be an early manifestation before the femoral head collapse, a reasonable method, such as placement of a graft within the proximal cortical layer, to prevent a collapse will be preferred [66].
Subchondral oxygen tension
Subchondral oxygen tension in ONFH hip was reported to be lower than the tension in the normal ones. Bone marrow mesenchymal stem cells in low oxygen (2%) culture had confirmed that finding, as well as preoperative scintigraphy and histological examination, when it was first proposed [67], [68]. The major cause of this phenomenon may be the interruption of blood supply, as in case of a femoral head fracture. Watanabe et al [69] tried to predict the collapse, but they did not find a direct correlation. They studied the oxygen tension of femoral neck fracture patients with necrosis. The authors used polarographic oxygen electrodes and an oxygen monitor to test multiple points from near the fracture line to the articular surface. They found that the oxygen tension of the necrosis group was lower at the articular surface than at the fracture line, compared with the normal group. This method produced measurable results; caused minimal damage, as the authors said; and better predicted the occurrence of necrosis, even a segmental collapse, especially in traumatic aetiology.
Hip cartilage thickness and diffusion coefficient analysis
Cartilage is essential for the occurrence of a collapse. The direct contact surface of both the acetabulum and the femoral head is the joint cartilage. Loss of cartilage, such as wearing or deformation, will lead to a collapse in the early phase. Leng et al [70] investigated patients with precollapse ONFH by imaging diagnosis, and analysed the hip cartilage thickness and the apparent diffusion coefficient. The apparent diffusion coefficient of hip cartilage in the study group was (15.23 ± 4.72) × 10−5 mm2/s and the hip joint cartilage thickness was 1.2 mm when the femoral head tended to collapse. Statistically significant differences were found between the control and study groups. Such types of methods were proposed initially for treating the Legg–Perthes–Calvé disease [71]. Multiple researchers believed that the thickness of the articular cartilage of the hip and the corresponding apparent diffusion coefficient measured by MRI are responsive to the articular cartilage changes, which can predict an early collapse of the ONFH, especially in the quantization of epiphysis development [72], [73].
Sclerotic rim analysis
A sclerotic rim around the osteonecrosis area is common in ONFH. Its biomechanical effect, however, has received little attention, and its natural formation process is also less studied. Sclerotic rim formation in osteonecrosis is a particular biomechanical product with ONFH progress, but also the signal structure of the necrosis healing [74]. Based on the clinical practice tips, a sclerotic rim is considered a biomechanical support to postpone or prevent the ONFH collapse [75]. Yu et al [76], [77] performed computed tomography of necrotic tissue of bilateral hip and calculated the proportion of sclerosis band in the proximal of the femoral head. Patients were divided into groups and noncollapse group, and the proportion of sclerosis bands and final collapse results are forward-looking analysed and assessed in predicted values. The results suggest that the proportion of sclerosis band in the femoral head is one kind of index for predicting a collapse in ONFH; 30% can be used as a critical value in clinical practice. When the value is higher than 30%, the risk is low, and vice versa. Chen et al [78] simulated density distribution in the Huiskes' bone remodelling model and calculated the maximal von Mises stress in the subchondral bone of the weight-bearing area of the femoral head. They similarly found that the sclerotic rim, similar to a compensatory load-bearing reinforcement, could act as an elastic modulus when the necrotic area is small.
Conclusion
Notably, the incidence of a collapse in ONFH becomes very high as the disease progresses. Considering that the collapse of the femoral head is the result of many biomechanical and biological factors that play a major role, forecasting a collapse event is very difficult. Once the femoral head collapses, hip osteoarthritis is inevitable, with high morbidity. If treatment is timely, orthopaedic surgeons should try to predict the possibility of the femoral head collapse and make intervention decisions quickly. Conservative treatments, such as core decompression surgery, fibular graft supporting, and stem cell implantation, in particular, should be implemented as soon as possible. We found that the current prediction methods, both in basic research and in clinical practice, were still measuring deviation and time delay. The main limitations of the different methods we discussed above were a lack of accuracy, impracticality, or both. Owing to this uncertainty, surgeons have a difficult choice to make and may be unwilling to adopt a different approach. This is the reason why many surgeons forego more conservative surgery or treatment. Therefore, even though a serious, comprehensive, and scientific system for predicting the femoral head collapse has not yet been developed, early preventive measures to delay or avoid total hip replacement procedures and to promote and control effective necrotic healing through conservative treatments are required for these to be viable options for patients and surgeons.
Conflicts of interest
The authors have no conflicts of interest relevant to this article.
Acknowledgements
This study was supported by grants from the National Nature Science Foundation of China (Grant no. 81302990), National Nature Science Foundation of China (Grant no. 81673999), Guangdong Natural Science Funds for Distinguished Young Scholars (2015A030306037), and Science and Technology Planning Project of Guangdong Province, China (2014A020221114).
References
- 1.Moya-Angeler J., Gianakos A.L., Villa J.C., Ni A., Lane J.M. Current concepts on osteonecrosis of the femoral head. World J Orthop. 2015;6:590–601. doi: 10.5312/wjo.v6.i8.590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang C., Peng J., Lu S. Summary of the various treatments for osteonecrosis of the femoral head by mechanism: a review. Exp Ther Med. 2014;8:700–706. doi: 10.3892/etm.2014.1811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen S.B., Hu H., Gao Y.S., He H.Y., Jin D.X., Zhang C.Q. Prevalence of clinical anxiety, clinical depression and associated risk factors in Chinese young and middle-aged patients with osteonecrosis of the femoral head. PLoS One. 2015;10:e0120234. doi: 10.1371/journal.pone.0120234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Motomura G., Yamamoto T., Yamaguchi R., Ikemura S., Nakashima Y., Mawatari T. Morphological analysis of collapsed regions in osteonecrosis of the femoral head. J Bone Joint Surg Br. 2011;93:184–187. doi: 10.1302/0301-620X.93B225476. [DOI] [PubMed] [Google Scholar]
- 5.Kerboul M., Thomine J., Postel M. d'Aubigne RM. The conservative surgical treatment of idiopathic aseptic necrosis of the femoral head. Bone Joint J. 1974;56:291–296. [PubMed] [Google Scholar]
- 6.Kubo T., Ueshima K., Saito M., Ishida M., Arai Y., Fujiwara H. Clinical and basic research on steroid-induced osteonecrosis of the femoral head in Japan. J Orthop Sci. 2016;21:407–413. doi: 10.1016/j.jos.2016.03.008. [DOI] [PubMed] [Google Scholar]
- 7.Karasuyama K., Yamamoto T., Motomura G., Sonoda K., Kubo Y., Iwamoto Y. The role of sclerotic changes in the starting mechanisms of collapse: a histomorphometric and FEM study on the femoral head of osteonecrosis. Bone. 2015;81:644–648. doi: 10.1016/j.bone.2015.09.009. [DOI] [PubMed] [Google Scholar]
- 8.Mont M.A., Cherian J.J., Sierra R.J., Jones L.C., Lieberman J.R. Nontraumatic osteonecrosis of the femoral head: where do we stand today? J Bone Joint Surg Am. 2015;97:1604–1627. doi: 10.2106/JBJS.O.00071. [DOI] [PubMed] [Google Scholar]
- 9.Floerkemeier T., Lutz A., Nackenhorst U., Thorey F., Waizy H., Windhagen H. Core decompression and osteonecrosis intervention rod in osteonecrosis of the femoral head: clinical outcome and finite element analysis. Int Orthop. 2011;35:1461–1466. doi: 10.1007/s00264-010-1138-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Conzemius M.G., Brown T.D., Zhang Y., Robinson R.A. A new animal model of femoral head osteonecrosis: one that progresses to human-like mechanical failure. J Orthop Res. 2002;20:303–309. doi: 10.1016/S0736-0266(01)00108-5. [DOI] [PubMed] [Google Scholar]
- 11.Hofstaetter J.G., Wang J., Yan J., Glimcher M.J. Changes in bone microarchitecture and bone mineral density following experimental osteonecrosis of the hip in rabbits. Cells Tissues Organs. 2007;184:138–147. doi: 10.1159/000099620. [DOI] [PubMed] [Google Scholar]
- 12.Chernetsky S.G., Mont M.A., LaPorte D.M., Jones L.C., Hungerford D.S., McCarthy E.F. Pathologic features in steroid and nonsteroid associated osteonecrosis. Clin Orthop Relat Res. 1999;368:149–161. [PubMed] [Google Scholar]
- 13.Seamon J., Keller T., Saleh J., Cui Q. The pathogenesis of nontraumatic osteonecrosis. Arthritis. 2012;2012:601763. doi: 10.1155/2012/601763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang G.J., Sweet D.E., Reger S.I., Thompson R.C. Fat-cell changes as a mechanism of avascular necrosis of the femoral head in cortisone-treated rabbits. J Bone Joint Surg Am. 1977;59:729–735. [PubMed] [Google Scholar]
- 15.Koo K.H., Kim R., Cho S.H., Song H.R., Lee G., Ko G.H. Angiography, scintigraphy, intraosseous pressure, and histologic findings in high-risk osteonecrotic femoral heads with negative magnetic resonance images. Clin Orthop Relat Res. 1994;308:127–138. [PubMed] [Google Scholar]
- 16.Hofmann S., Engel A., Neuhold A., Leder K., Kramer J., Plenk H. Bone-marrow oedema syndrome and transient osteoporosis of the hip. An MRI-controlled study of treatment by core decompression. J Bone Joint Surg Br. 1993;75:210–216. doi: 10.1302/0301-620X.75B2.8444939. [DOI] [PubMed] [Google Scholar]
- 17.Ohzono K., Saito M., Sugano N., Takaoka K., Ono K. The fate of nontraumatic avascular necrosis of the femoral head: a radiologic classification to formulate prognosis. Clin Orthop Relat Res. 1992;277:73–78. [PubMed] [Google Scholar]
- 18.Chen C.C., Lin C.L., Chen W.C., Shih H.N., Ueng S.W., Lee M.S. Vascularized iliac bone-grafting for osteonecrosis with segmental collapse of the femoral head. J Bone Joint Surg Am. 2009;91:2390–2394. doi: 10.2106/JBJS.H.01814. [DOI] [PubMed] [Google Scholar]
- 19.Mont M.A., Zywiel M.G., Marker D.R., McGrath M.S., Delanois R.E. The natural history of untreated asymptomatic osteonecrosis of the femoral head. J Bone Joint Surg Am. 2010;92:2165–2170. doi: 10.2106/JBJS.I.00575. [DOI] [PubMed] [Google Scholar]
- 20.Steinberg M., Hayken G., Steinberg D. A new method for evaluation and staging of avascular necrosis of the femoral head. In: Arlet J., Ficat R.P., Hungerford D.S., editors. Bone circulation. Williams and Wilkins; Baltimore: 1984. pp. 389–403. [Google Scholar]
- 21.Mont M.A., Hungerford D.S. Non-traumatic avascular necrosis of the femoral head. J Bone Joint Surg Am. 1995;77:459–474. doi: 10.2106/00004623-199503000-00018. [DOI] [PubMed] [Google Scholar]
- 22.Sugano N., Takaoka K., Ohzono K., Matsui M., Masuhara K., Ono K. Prognostication of nontraumatic avascular necrosis of the femoral head: significance of location and size of the necrotic lesion. Clin Orthop Relat Res. 1994;303:155–164. [PubMed] [Google Scholar]
- 23.Koo K.H., Kim R., Ko G.H., Song H.R., Jeong S.T., Cho S.H. Preventing collapse in early osteonecrosis of the femoral head. A randomised clinical trial of core decompression. Bone Joint J. 1995;77:870–874. [PubMed] [Google Scholar]
- 24.Lafforgue P., Dahan E., Chagnaud C.H., Schiano A., Kasbarian M., Acquaviva P.C. Early-stage avascular necrosis of the femoral head: MR imaging for prognosis in 31 cases with at least 2 years of follow-up. Radiology. 1993;187:199–204. doi: 10.1148/radiology.187.1.8451413. [DOI] [PubMed] [Google Scholar]
- 25.Beltran J., Knight C.T., Zuelzer W.A., Morgan J.P., Shwendeman L.J., Chandnani V.P. Core decompression for avascular necrosis of the femoral head: correlation between long-term results and preoperative MR staging. Radiology. 1990;175:533–536. doi: 10.1148/radiology.175.2.2326478. [DOI] [PubMed] [Google Scholar]
- 26.Koo K.H., Kim R.O. Quantifying the extent of osteonecrosis of the femoral head. A new method using MRI. Bone Joint J. 1995;77:875–880. [PubMed] [Google Scholar]
- 27.Steinberg M.E., Bands R.E., Parry S., Hoffman E., Chan T., Hartman K.M. Does lesion size affect the outcome in avascular necrosis? Clin Orthop Relat Res. 1999;367:262–271. [PubMed] [Google Scholar]
- 28.Shimizu K., Moriya H., Akita T., Sakamoto M., Suguro T. Prediction of collapse with magnetic resonance imaging of avascular necrosis of the femoral head. J Bone Joint Surg Am. 1994;76:215–223. doi: 10.2106/00004623-199402000-00007. [DOI] [PubMed] [Google Scholar]
- 29.Chen C.H., Chang J.K., Huang K.Y., Hung S.H., Lin G.T., Lin S.Y. Core decompression for osteonecrosis of the femoral head at pre-collapse stage. Kaohsiung J Med Sci. 2000;16:76–82. [in Chinese] [PubMed] [Google Scholar]
- 30.Zhao F.C., Li Z.R., Zhang F.N., Ma C., Xiong C.Z. Prediction of collapse with magnetic resonance imaging of avascular necrosis of the femoral head. J Pract Orthop. 2005;11:108–112. [in Chinese] [Google Scholar]
- 31.Ha Y.C., Jung W.H., Kim J.R., Seong N.H., Kim S.Y., Koo K.H. Prediction of collapse in femoral head osteonecrosis: a modified Kerboul method with use of magnetic resonance images. J Bone Joint Surg. 2006;88(Suppl. 3):35–40. doi: 10.2106/JBJS.F.00535. [in Chinese] [DOI] [PubMed] [Google Scholar]
- 32.Connolly P., Weinstein S.L. The course and treatment of avascular necrosis of the femoral head in developmental dysplasia of the hip. Acta Orthop Traumatol Turc. 2006;41:54–59. [in Turkish] [PubMed] [Google Scholar]
- 33.Sugano N., Ohzono K., Masuhara K., Takaoka K., Ono K. Prognostication of osteonecrosis of the femoral head in patients with systemic lupus erythematosus by magnetic resonance imaging. Clin Orthop Relat Res. 1994;305:190–199. [PubMed] [Google Scholar]
- 34.Takatori Y, Nakamura S, Morimoto SA. Classification of idiopathic osteonecrosis of the femoral head on the basis of the site and extent of the site and extent of the necrotic lesion. vol. 35. In: The 7th International Symposium on Bone Necrosis, 1996.
- 35.Sakamoto M.A., Shimizu K., Iida S.A., Akita T.O., Moriya H.I., Nawata Y.A. Osteonecrosis of the femoral head. Bone Joint J. 1997;79:213–219. doi: 10.1302/0301-620x.79b2.7179. [DOI] [PubMed] [Google Scholar]
- 36.Ito H., Matsuno T., Kaneda K. Prognosis of early stage avascular necrosis of the femoral head. Clin Orthop Relat Res. 1999;358:149–157. [PubMed] [Google Scholar]
- 37.Manenti G., Altobelli S., Pugliese L., Tarantino U. The role of imaging in diagnosis and management of femoral head avascular necrosis. Clin Cases Miner Bone Metab. 2015;12(Suppl. 1):31–38. doi: 10.11138/ccmbm/2015.12.3s.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zalavras C.G., Lieberman J.R. Osteonecrosis of the femoral head: evaluation and treatment. J Am Acad Orthop Surg. 2014;22:455–464. doi: 10.5435/JAAOS-22-07-455. [DOI] [PubMed] [Google Scholar]
- 39.Kokubo T., Takatori Y., Ninomiya S., Nakamura T., Kamogawa M. Magnetic resonance imaging and scintigraphy of avascular necrosis of the femoral head: prediction of subsequent segmental collapse. Clin Orthop Relat Res. 1992;277:54–60. [PubMed] [Google Scholar]
- 40.Takatori Y.O., Kokubo T.A., Ninomiya S.E., Nakamura S.H., Morimoto S., Kusaba I. Avascular necrosis of the femoral head. Natural history and magnetic resonance imaging. Bone Joint J. 1993;75:217–221. doi: 10.1302/0301-620X.75B2.8444940. [DOI] [PubMed] [Google Scholar]
- 41.Koo K.H., Ahn I.O., Kim R., Song H.R., Jeong S.T., Na J.B. Bone marrow edema and associated pain in early stage osteonecrosis of the femoral head: prospective study with serial MR images. Radiology. 1999;213:715–722. doi: 10.1148/radiology.213.3.r99dc06715. [DOI] [PubMed] [Google Scholar]
- 42.Bassett L.W., Mirra J.M., Cracchiolo A.N., III, Gold R.H. Ischemic necrosis of the femoral head: correlation of magnetic resonance imaging and histologic sections. Clin Orthop Relat Res. 1987;223:181–187. [PubMed] [Google Scholar]
- 43.Wang G., Zhao G., Zhang X. Magnetic resonance imaging with multiplane histomorphometry in avascular necrosis of femoral head: analysis of trabeculae. Zhonghua Yi Xue Za Zhi. 1998;78:467–469. [in Chinese] [PubMed] [Google Scholar]
- 44.Iwasada S., Hasegawa Y., Iwase T., Kitamura S., Iwata H. Bone scintigraphy and magnetic resonance imaging after transtrochanteric rotational osteotomy. Skelet Radiol. 1999;28:251–259. doi: 10.1007/s002560050511. [DOI] [PubMed] [Google Scholar]
- 45.Nishii T., Sugano N., Ohzono K., Sakai T., Sato Y., Yoshikawa H. Significance of lesion size and location in the prediction of collapse of osteonecrosis of the femoral head: a new three-dimensional quantification using magnetic resonance imaging. J Orthop Res. 2002;20:130–136. doi: 10.1016/S0736-0266(01)00063-8. [DOI] [PubMed] [Google Scholar]
- 46.Zhao W.P., Lin F., Lu Q.P., Wu Z.H., Shi Z.C. 3D reconstruction and FEA in the prediction of osteonecrosis collapse of the femoral head. Chin J Biomed Eng. 2005;24:784–787. [Google Scholar]
- 47.Kang B., Tang S.T., Yang H. Study on application of bone mineral density in prediction of collapse of femoral head after avascular necrosis. J Pract Orthop. 2009;01:30–32. [in Chinese] [Google Scholar]
- 48.Wang C., Wang X., Xu X.L., Yuan X.L., Gou W.L., Wang A.Y. Bone microstructure and regional distribution of osteoblast and osteoclast activity in the osteonecrotic femoral head. PLoS One. 2014;9:e96361. doi: 10.1371/journal.pone.0096361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang L., Zhang L., Pan H., Peng S., Zhao X., Lu W.W. Abnormal subchondral bone microstructure following steroid administration is involved in the early pathogenesis of steroid-induced osteonecrosis. Osteoporos Int. 2016;27:153–159. doi: 10.1007/s00198-015-3225-8. [DOI] [PubMed] [Google Scholar]
- 50.Kumar M.N., Belehalli P., Ramachandra P. PET/CT study of temporal variations in blood flow to the femoral head following low-energy fracture of the femoral neck. Orthopedics. 2014;37:e563–e570. doi: 10.3928/01477447-20140528-57. [DOI] [PubMed] [Google Scholar]
- 51.Murphey M.D., Foreman K.L., Klassen-Fischer M.K., Fox M.G., Chung E.M., Kransdorf M.J. From the radiologic pathology archives imaging of osteonecrosis: radiologic–pathologic correlation. Radiographics. 2014;34:1003–1028. doi: 10.1148/rg.344140019. [DOI] [PubMed] [Google Scholar]
- 52.Win A.Z., Aparici C.M. Non-traumatic radiation-induced avascular necrosis of the femoral neck. QJM. 2015;108:257–258. doi: 10.1093/qjmed/hcu190. [DOI] [PubMed] [Google Scholar]
- 53.Sun H.J., Yuan X.M., Li S.L. Diagnostic analysis of 42 cases of avascular necrosis of the femoral head by bone scintigraphy. Chin J Misdiagn. 2007;3:580–581. [in Chinese] [Google Scholar]
- 54.Sakagoshi D., Kabata T., Umemoto Y., Sakamoto J., Tomita K. A mechanical analysis of femoral resurfacing implantation for osteonecrosis of the femoral head. J Arthroplasty. 2010;25:1282–1289. doi: 10.1016/j.arth.2009.09.002. [DOI] [PubMed] [Google Scholar]
- 55.Gou W.L., Lu Q., Wang X., Wang Y., Peng J., Lu S.B. Key pathway to prevent the collapse of femoral head in osteonecrosis. Eur Rev Med Pharmacol Sci. 2015;19:2766–2774. [PubMed] [Google Scholar]
- 56.Zhou G.Q., Pang Z.H., Chen Q.Q., He W., Chen Z.Q., Chen L.L. Reconstruction of the biomechanical transfer path of femoral head necrosis: a subject-specific finite element investigation. Comput Biol Med. 2014;52:96–101. doi: 10.1016/j.compbiomed.2014.04.002. [DOI] [PubMed] [Google Scholar]
- 57.Zhou G., Zhang Y., Zeng L., He W., Pang Z., Chen X. Should thorough debridement be used in fibular allograft with impaction bone grafting to treat femoral head necrosis: a biomechanical evaluation. BMC Musculoskelet Disord. 2015;16:140. doi: 10.1186/s12891-015-0593-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cui X., Zhao D.W., Gu C.J. Biomechanical study on predicting the collapse of ischemic necrosis of the femoral head. Chin J Clin Anat. 2005;23:193–198. [in Chinese] [Google Scholar]
- 59.Fang B., He W., Zhan L., Zhang Q.W., Wei Q.S., Chen Z.Q. Finite element analysis of stress distribution over femoral head necrosis zones in different necrosis areas. J Tradit Chin Orthop Traumatol. 2012;24:10–16. [in Chinese] [Google Scholar]
- 60.Even-Sapir E., Mishani E., Flusser G., Metser U. vol. 37, No. 6. November 30, 2007. 18 F-fluoride positron emission tomography and positron emission tomography/computed tomography; pp. 462–469. (Seminars in nuclear medicine). [DOI] [PubMed] [Google Scholar]
- 61.Kobayashi N., Inaba Y., Tateishi U., Yukizawa Y., Ike H., Inoue T. New application of 18F-fluoride PET for the detection of bone remodeling in early-stage osteoarthritis of the hip. Clin Nucl Med. 2013;38:e379–e383. doi: 10.1097/RLU.0b013e31828d30c0. [DOI] [PubMed] [Google Scholar]
- 62.Dasa V., Adbel-Nabi H., Anders M.J., Mihalko W.M. F-18 fluoride positron emission tomography of the hip for osteonecrosis. Clin Orthop Relat Res. 2008;466:1081–1086. doi: 10.1007/s11999-008-0219-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kubota S., Inaba Y., Kobayashi N., Tateishi U., Ike H., Inoue T. Prediction of femoral head collapse in osteonecrosis using 18F-fluoride positron emission tomography. Nucl Med Commun. 2015;36:596–603. doi: 10.1097/MNM.0000000000000284. [DOI] [PubMed] [Google Scholar]
- 64.Schiepers C., Broos P., Miserez M., Bormans G., De Roo M. Measurement of skeletal flow with positron emission tomography and 18F-fluoride in femoral head osteonecrosis. Arch Orthop Trauma Surg. 1998;118:131–135. doi: 10.1007/s004020050332. [DOI] [PubMed] [Google Scholar]
- 65.Volokh K.Y., Yoshida H., Leali A., Fetto J.F., Chao E.Y. Prediction of femoral head collapse in osteonecrosis. J Biomech Eng. 2006;128:467–470. doi: 10.1115/1.2187050. [DOI] [PubMed] [Google Scholar]
- 66.Hattori Y. Vascularized bone graft from the supracondylar region of the femur. Microsurgery. 2009;29:379–384. doi: 10.1002/micr.20671. [DOI] [PubMed] [Google Scholar]
- 67.Kiaer T.H., Pedersen N.W., Kristensen K.D., Starklint H.E. Intra-osseous pressure and oxygen tension in avascular necrosis and osteoarthritis of the hip. Bone Joint J. 1990;72:1023–1030. doi: 10.1302/0301-620X.72B6.2246284. [DOI] [PubMed] [Google Scholar]
- 68.Fan L., Liu R., Li J., Shi Z., Dang X., Wang K. Low oxygen tension enhances osteogenic potential of bone marrow-derived mesenchymal stem cells with osteonecrosis-related functional impairment. Stem Cells Int. 2015 doi: 10.1155/2015/950312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Watanabe Y., Terashima Y., Takenaka N., Kobayashi M., Matsushita T. Prediction of avascular necrosis of the femoral head by measuring intramedullary oxygen tension after femoral neck fracture. J Orthop Trauma. 2007;21:456–461. doi: 10.1097/BOT.0b013e318126bb56. [DOI] [PubMed] [Google Scholar]
- 70.Leng X.M., Jiang S.P., Huang Y., Zeng D.H., Feng X., Zhao M. Pre-collapse of articular cartilage of femoral head necrosis of MRI measurement of thickness and surface analysis of diffusion coefficient values. Chin J CT MRI. 2015;07:101–103. [in Chinese] [Google Scholar]
- 71.Boutault J.R., Baunin C., Bérard E., Vial J., Labarre D., Domenech C. Diffusion MRI of the neck of the femur in Legg–Calve–Perthes disease: a preliminary study. Diagn Interv Imaging. 2013;94:78–83. doi: 10.1016/j.diii.2012.10.003. [DOI] [PubMed] [Google Scholar]
- 72.Ozel B.D., Ozel D., Ozkan F., Halefoglu A.M. Diffusion-weighted magnetic resonance imaging of femoral head osteonecrosis in two groups of patients: Legg–Perthes–Calve and avascular necrosis. La Radiol Med. 2016;121:206–213. doi: 10.1007/s11547-015-0589-y. [DOI] [PubMed] [Google Scholar]
- 73.Baunin C., Sanmartin-Viron D., Accadbled F., Sans N., Vial J., Labarre D. Prognosis value of early diffusion MRI in Legg Perthes Calvé disease. Orthop Traumatol Surg Res. 2014;100:317–321. doi: 10.1016/j.otsr.2013.12.025. [DOI] [PubMed] [Google Scholar]
- 74.Nakai T., Masuhara K., Nakase T., Sugano N., Ohzono K., Ochi T. Pathology of femoral head collapse following transtrochanteric rotational osteotomy for osteonecrosis. Arch Orthop Trauma Surg. 2000;120:489–492. doi: 10.1007/s004020000144. [DOI] [PubMed] [Google Scholar]
- 75.Liu Z.H., Li Z.R., Sun W., Zhang N.F., Guo W.S. Risk factors for collapse in patients with osteonecrosis of bilateral femoral heads: retrospective analysis based on MRI and CT. J Clin Rehabil Tissue Eng Res. 2008;22:4249–4252. [in Chinese] [Google Scholar]
- 76.Yu T., Xie L., Zhang Z., Ke X., Liu Y. Prediction of osteonecrosis collapse of the femoral head based on the proportion of the proximal sclerotic rim. Int Orthop. 2015;39:1045–1050. doi: 10.1007/s00264-014-2602-9. [DOI] [PubMed] [Google Scholar]
- 77.Yu T., Xie L., Chu F. A sclerotic rim provides mechanical support for the femoral head in osteonecrosis. Orthopedics. 2015;38:e374–e379. doi: 10.3928/01477447-20150504-53. [DOI] [PubMed] [Google Scholar]
- 78.Chen Z., Xu Y., Qi Z., Zho J. The formation and function of the sclerosis rim in the femoral head: a biomechanical point of view. Med Eng Phys. 2015;37:1125–1132. doi: 10.1016/j.medengphy.2015.09.005. [DOI] [PubMed] [Google Scholar]