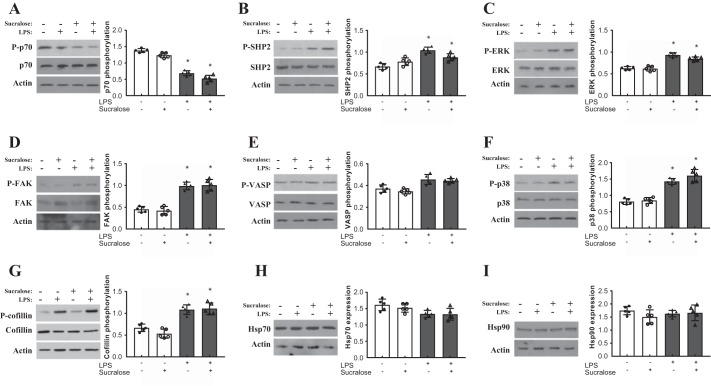
Fig. 7.
Role of sucralose on LPS-mediated signaling is independent of several key molecules. Rat LMVECs were treated in the presence or absence of LPS (1 µg/ml) and sucralose (0.1 mM) for 24 h. Phosphorylation of HSP70 (A), SHP2 (B), ERK (C), FAK (D), VASP (E), p38 (F), and cofillin (G) was assessed in whole-cell lysates by immunoblot analysis with an antibody specific to each phosphorylated protein. Blots were stripped and reprobed for total protein expression and actin as a loading control. Total protein expression of HSP70 (H) and HSP90 (I) was also assessed in whole-cell lysates, followed strip and reprobe of blots for actin as a loading control. Representative blots are shown. n = 6. Data are expressed as means ± SD. *P < 0.05 vs. vehicle for LPS.