Significance
Understanding the key drivers of animal movement is crucial to assist in mitigating adverse impacts of anthropogenic activities on marine megafauna. We found that movement patterns of marine megafauna are mostly independent of their evolutionary histories, differing significantly from patterns for terrestrial animals. We detected a remarkable convergence in the distribution of speed and turning angles across organisms ranging from whales to turtles (epitome for the slowest animals on land but not at sea). Marine megafauna show a prevalence of movement patterns dominated by search behavior in coastal habitats compared with more directed, ballistic movement patterns when the animals move across the open ocean. The habitats through which they move will therefore need to be considered for effective conservation.
Keywords: global satellite tracking, probability density function, root-mean-square, turning angles, displacements
Abstract
The extent of increasing anthropogenic impacts on large marine vertebrates partly depends on the animals’ movement patterns. Effective conservation requires identification of the key drivers of movement including intrinsic properties and extrinsic constraints associated with the dynamic nature of the environments the animals inhabit. However, the relative importance of intrinsic versus extrinsic factors remains elusive. We analyze a global dataset of ∼2.8 million locations from >2,600 tracked individuals across 50 marine vertebrates evolutionarily separated by millions of years and using different locomotion modes (fly, swim, walk/paddle). Strikingly, movement patterns show a remarkable convergence, being strongly conserved across species and independent of body length and mass, despite these traits ranging over 10 orders of magnitude among the species studied. This represents a fundamental difference between marine and terrestrial vertebrates not previously identified, likely linked to the reduced costs of locomotion in water. Movement patterns were primarily explained by the interaction between species-specific traits and the habitat(s) they move through, resulting in complex movement patterns when moving close to coasts compared with more predictable patterns when moving in open oceans. This distinct difference may be associated with greater complexity within coastal microhabitats, highlighting a critical role of preferred habitat in shaping marine vertebrate global movements. Efforts to develop understanding of the characteristics of vertebrate movement should consider the habitat(s) through which they move to identify how movement patterns will alter with forecasted severe ocean changes, such as reduced Arctic sea ice cover, sea level rise, and declining oxygen content.
Unifying theoretical frameworks that explain general principles of animal life-history (1), optimal foraging (2, 3), and metabolic scaling in organisms (4, 5) facilitate the interpretation of data and the generation of testable hypotheses. Animal movement accounts for most of the energy budgets of vertebrates because it underpins critical components of their behavior, such as feeding and mating. Following the challenge posed by Aristotle millennia ago in De Motu Animalium (On the Movement of Animals) (6), efforts have been made to develop a unifying framework to study movement (7). [“Now we must consider in general the common reason for moving with any movement whatever (for some animals move by flying, some by swimming, some by stepping, some in other comparable ways).” (ref. 6, p. 24)] Such efforts have provided clarification that the primary challenge for understanding animal movement lies in the identification of the key external factors, internal states, and the motion and navigation capabilities influencing movement (7). It is also known that animal movement patterns are underpinned by common principles, such as “optimal” resource exploitation by predators (“optimality paradigm”; refs. 2, 3, 8, and 9) or the use of more efficient search trajectories (“random” paradigm; refs. 10–12). Overall, animal movement patterns have been attributed to extrinsic factors, including the dynamic nature of the environments they inhabit and constrained by intrinsic properties (13–19), including allometric and metabolic scaling with day or home range and locomotion speed, particularly for terrestrial animals (4, 15, 20–23). However, the relative importance of extrinsic versus intrinsic properties in determining the observed patterns of movement of free-ranging animals remains ambiguous. To effectively partition the relative contributions of extrinsic versus intrinsic factors and effectively investigate whether a unifying framework exists irrespective of location, scale of movement, and stage or phase, a large-scale comparison across multiple species is needed (24, 25).
Rapid technological developments in animal-attached electronic tags (telemetry/biologging) have generated large tracking datasets across an array of marine vertebrates, now available for multiple regions, temporal scales, and habitats across the globe. Such large datasets provide the foundational information required to discover commonalities in movement patterns across species and environments and to assess the influence of a range of intrinsic and extrinsic factors. Because marine vertebrates have diverse life histories and include all extant vertebrate classes except Amphibia, they provide an ideal group for the exploration of the underlying principles that might govern animal movement. Moreover, marine vertebrates range broadly in their movement patterns, from species with small home ranges (centimeters to kilometers) to highly migratory animals traveling hundreds to thousands of kilometers while crossing entire ocean basins (26–28). For these reasons, answering questions about marine animal movement will have broad-reaching application in understanding movement in species from terrestrial vertebrates to aquatic invertebrates (29). Moreover, marine vertebrates include many threatened species that are particularly vulnerable to changing environments (e.g., polar bears and penguins) (30) or to extractive anthropogenic activities (e.g., whales, sharks, and seals), as well as species with important economic value to human societies (31, 32). Hence, understanding how marine vertebrates move is critical to broadly understand the mechanisms of animal movement as well as to assist developing effective conservation measures and predicting the potential impacts of global change on populations.
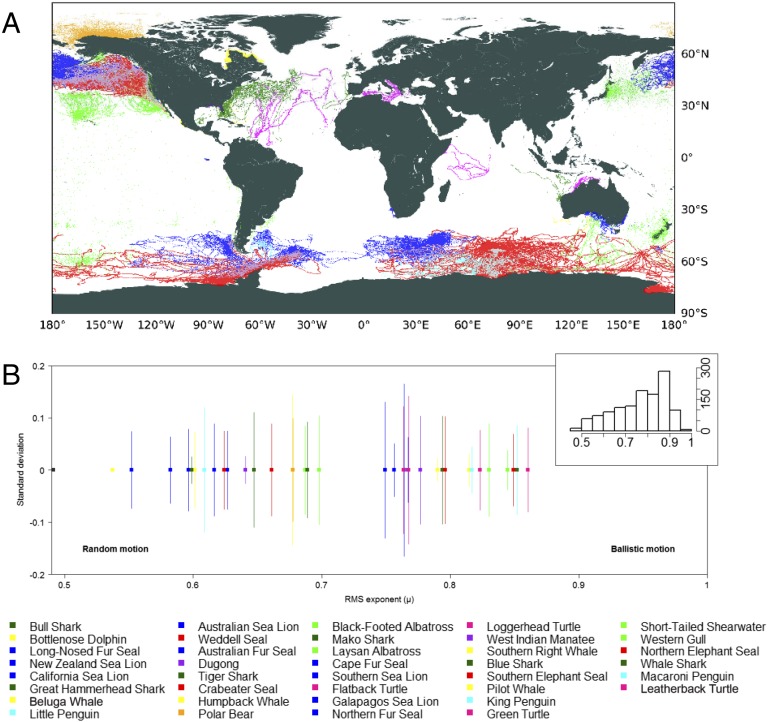
Here, we synthesize movement data from the largest satellite telemetry dataset yet assembled at a global scale for large marine vertebrate species (termed “megafauna”) to quantify the relative importance of both extrinsic and intrinsic factors as drivers of movement and identify unifying patterns in marine megafauna movement. Our dataset includes species that fly, swim, and walk/paddle, with distributional ranges varying across tropical, temperate, and polar regions, and comprising sharks, turtles, flying and swimming birds, true and eared seals, cetaceans, sirenians, and polar bears (Fig. 1A). We analyzed individual movement by characterizing horizontal displacements as the shortest great-circle distances between two consecutive locations and the turning angles between them. We tested for differences in these attributes among taxonomic groups (taxa, family, species), allometric scaling (body length and mass), life history traits (e.g., breeding and foraging strategies), energy requirements, as well as locomotion mode, region (polar, temperate, tropical), and coastal affinity, defined as the fraction of displacements within the 0–150 m depth range (here referred to as “coastal ocean”) (see Materials and Methods for details and SI Appendix, Tables S1–S3).
Fig. 1.
Representation of the global tracking dataset and scaling properties for all species analyzed. (A) Global map with trajectories obtained by satellite tracking for all 50 species. (B) The scaling exponents (μ) obtained from the root-mean-square (dRMS) analysis of displacements for all species analyzed (species names indicated from left to right in caption; squares indicate mean values, and bars show the SD). Histogram in Inset shows the number of individuals for the range of scaling exponents. Long-nosed fur seal: common name for the South Australian population of New Zealand fur seal. Colors represent each of the nine guilds with data: cetaceans (yellow), eared seals (blue), flying birds (green), penguins (cyan), polar bears (orange), sharks (dark green), sirenians (purple), true seals (red), and turtles (pink).
Results
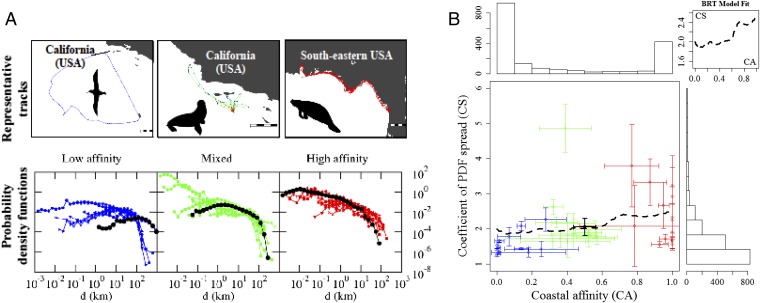
The mean displacements per day (d in km/d; effective speed), referred to in the terrestrial animal movement literature as “day range” (DR) (21) or “daily movement range” (20), were mostly independent of body length and mass (SI Appendix, Fig. S1) both among and within species groups. Exceptions include true seals (body length only) and turtles, but the latter is simply associated with the different mode of foraging of the largest turtle considered, the leatherback turtle, which constantly travels large distances in search of prey. The other turtle species included in the analysis tend to be neritic as adults, living most of their lives in shallow coastal waters where they move little. The root-mean-square analysis of displacements (dRMS) scaled with time as a power law (dRMS ∼ Tμ) with exponents (μ) mostly above the value of 0.5 (commonly associated with Brownian motion) (Fig. 1B), indicating that most individuals moved superdiffusively—that is, faster than expected in a normal diffusion process. Using predetermined time windows of 1 d, we compared the probability density functions (PDFs) of the observed displacements for each individual using a dimensionless coefficient of PDF spread (CS), defined as the ratio between the second moment (average square displacement) and the square of the first moment (square of the average displacement). Our CS can be used for comparison across all individuals irrespective of scale and provides an estimate of the spread of the resulting PDF normalized by the square of the average displacement (Fig. 2A). Generally, CS >> 1 indicates wide distributions with heavy tails, such as a power law or lognormal. Lower values generally highlight a narrower range of displacements identified in the tracked movement resulting in a smaller spread of the PDFs. Our CS results show high variability among individuals within and among species (Fig. 2B and SI Appendix, Table S4), revealing substantial within-species variability in movement patterns, with increasing mean coefficient of variation from 22.94%, 38.10%, and 40.37% for species grouped within low, mixed, and high coastal affinity, respectively (Fig. 2B). Lower CS values generally represented simpler, more linear paths, while higher values represented more varied, complex movement patterns (Fig. 2A).
Fig. 2.
Results of the analysis of displacements. (A) PDF of displacements (d, km) at the species level with 1-d time windows for species with low (mean < 0.3; Left), mixed (Center), and high (mean > 0.7; Right) coastal affinity (Bottom), and example tracks for each group (Top; black and white scale bars represent 100 km; black dotted lines: PDF for the example track shown). (B) Relationship between coefficient of PDF spread (CS) and coastal affinity (CA) obtained from the BRTs (dashed black line; also shown in the Top Right Inset). To the top and right are histograms of CA and CS, respectively. Outlier green point: western gulls (0 ≤ CA ≤ 1; SI Appendix, Table S4). Average coefficients of variation: 22.94%, 38.10%, and 40.37% for CS, and 243.55%, 85.93%, and 11.48% for CA for low (blue), mixed (green), and high (red) CA species, respectively. Solid black: mean ± SD among all species with a coefficient of variation of 37.20% for CS and 39.51% CA.
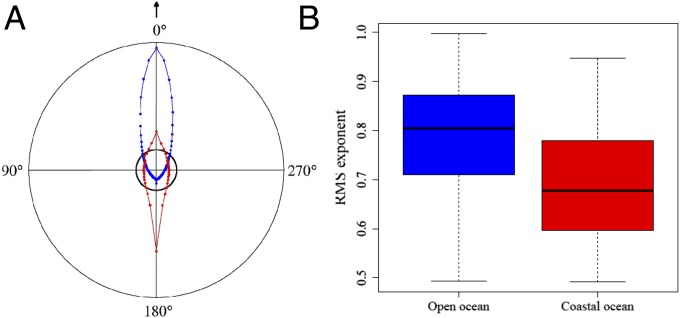
Model fits from boosted regression trees (BRTs; ref. 33) to the resulting CSs for all individuals showed that species group and coastal affinity had the highest relative importance (74.0% and 21.5%, respectively) and identified an interaction (size = 36.0) between these two variables (SI Appendix, Fig. S2). This interaction highlights that the different movement patterns in the coastal and open ocean are not uniquely a species-specific trait and partly accounts for the high variability in movement patterns among individuals within species (Fig. 2 and SI Appendix, Fig. S2). Moreover, life-history traits, such as breeding and foraging strategies, as well as allometric traits, such as body mass and length, were not shown to greatly influence CS and were mostly removed during the simplification procedure in the BRTs. Based on our modeling results, CS increases with greater coastal affinity (Fig. 2B), indicating a larger range of displacements observed when individuals move mostly through coastal areas (i.e., including small and very large displacements). An association between coastal affinity and the dRMS exponent (with 0.5–1.0 indicating normal random to superdiffusive, more directed, ballistic motion) was also detected. Higher dRMS exponents were found for all displacements taking place in the open ocean, where depths ≥ 150 m (dRMS exponent = 0.791–0.112 × ocean, where ocean is “open ocean” or “coastal ocean”, P < 0.001 for the linear model) (Fig. 3). This result is congruent with simple, extensive, and directed movements in that habitat. It is also supported by the finding of prevailing frequency of angles of 0°—that is, more directed, forward movement patterns in open ocean (depths ≥150 m), and less directed patterns for displacements in the coastal ocean (depths <150 m) with higher frequency of lateral and backward angles (Fig. 3 and SI Appendix, Fig. S3).
Fig. 3.
Comparison of movement patterns in open and coastal oceans. The classification into open or coastal was based on the depth at which the displacements occurred with depth ≤ 150 m classified as coastal ocean. (A) Distribution of turning angles (arrow points to 0°) in open (blue) and coastal oceans (red). The circular plot reveals high frequency of 0° angles in open ocean and a large number of angles between 90° and 270° angles (peaking at 180°; i.e., returns) in coastal oceans. Black inner circle: uniform distribution of angles. (B) Boxplot of dRMS exponents (μ) for individuals showing coastal affinity below (blue) and above (red) 0.5 (μ = 0.784–0.085 × coastal ocean; P < 0.001).
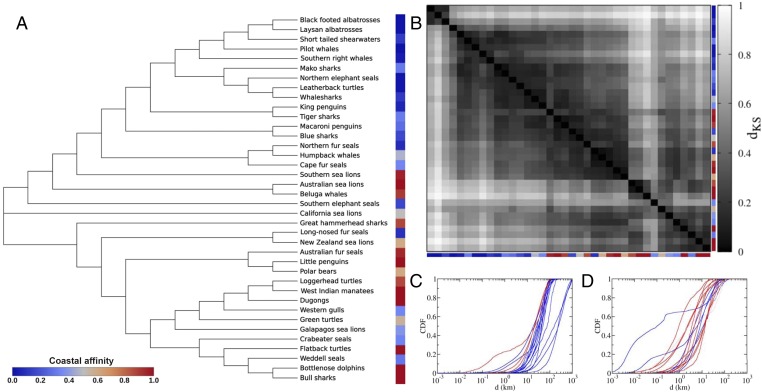
The Kolmogorov–Smirnov analysis of the distances between the cumulative distributions of displacements for each species pair was used to compute a dendrogram that resulted in two main clusters above and below California sea lions (a species with mixed coastal affinity). The split of clusters was consistent with a split between species moving in coastal and open oceans (i.e., high and low coastal affinities as shown in the color scale for Fig. 4A). The resulting dendrogram was unrelated to phylogeny, such that closely related species were no more similar in their displacement patterns than were distantly related ones. This result further reinforces that habitat structure is an important driver of the movement patterns among marine megafauna and reiterates that differences are not fully dependent on intrinsic traits.
Fig. 4.
Analysis of distances between the CDFs of displacements for each pair of species. The colors shown correspond to the classification of each species as having high (red) or low coastal affinity (blue) based on the proportion of their observed displacements occurring completely within coastal ocean for each individual of the same species (SI Appendix, Table S4). (A) Dendrogram obtained from the distance matrix derived from the Kolmogorov–Smirnov analysis showing two main branches (anchored by the line for California sea lions) broadly associated with low (<0.5; upper branch) and high (>0.5, lower branch) coastal affinity. (B) Distance matrix (mirror image from diagonal) with darker colors indicating short distances (dKS) between the species’ CDFs. C and D show the CDF of displacements for species in the upper and lower branch of the dendrogram, respectively, highlighting distinct displacement regions (d in the x axis) for the curves relative to species occurring mostly on open and coastal oceans, respectively.
Discussion
Our integrated, multispecies study revealed that differences in movement patterns of large marine vertebrates are primarily defined by the species to which they belong (74% relative influence) but underpinned by a strong interaction with the habitat through which the animals move (open or coastal ocean; 21% relative influence). This interaction partly accounts for the large variability in movement patterns among individuals of the same species (Fig. 2 and SI Appendix, Fig. S6) and is likely related to a combination of directed- and resident-type movements that can occur over the course of an individual’s track in association with different behaviors (e.g., transiting and foraging, resting, breeding) (34). Here, we used an analysis of displacements with no prior assumptions for the movement patterns observed and show that coastal and open ocean habitats directly influence the horizontal movement patterns of marine megafauna potentially in association with habitat complexity and related prey availability. The patterns we found emerged consistently across a diverse array of marine vertebrates and locomotion modes, confirming that habitat structure is a powerful driver of movement patterns. Species with movements that occurred mostly in coastal environments displayed a greater variety of displacement lengths (indicated by the generally higher CS; Fig. 2 and SI Appendix, Fig. S6), regardless of the intrinsic factors related to each species. In contrast, species moving mostly off-shelf in deep, oceanic habitat conformed to a relatively narrower range of displacements, which indicated less variability in displacement lengths (generally larger). We suggest that this difference in behavior between on- and off-shelf movements is related to habitat complexity, with open water habitats off the continental shelves being comparatively less complex (i.e., more homogeneous physical habitat) despite their highly dynamic nature. Open ocean species tend to use oceanographic features, such as fronts, eddies, and currents, as foraging and movement cues (e.g., ref. 35). This difference may explain the convergence of movement behaviors characterized by more directed movement with generally higher dRMS exponents among species when moving off the continental shelf. In contrast, animals moving over the shelf and in the coastal ocean experience a much wider variety of structurally complex habitats (e.g., reef, seagrass) that support a diverse suite of varying resources (e.g., prey, refuges) and threats (e.g., predators, human disturbance), stimulating more complex movement patterns and covering a larger range of displacements (Fig. 2 and SI Appendix, Fig. S6). This complex mix of features provides a rich and diverse array of opportunities for foraging, breeding, and other behaviors (36). Previous studies of movement have also revealed different patterns exhibited by marine fish and foraging albatrosses in a manner consistent with prey distribution between coastal shelf and oceanic areas (37, 38).
Our results reveal a remarkable convergence in movement patterns among a large range of marine vertebrates, departing from those reported for terrestrial animals in that the patterns we detected were independent of body length and mass (despite these ranging 10 orders of magnitude among the species studied). By contrast, for a variety of terrestrial species, the DR is known to scale with body mass, following power laws with exponents around 0.25 (20, 21), with slight differences in the scaling for different taxonomic groups associated with diet types and foraging habitats (21). Our finding suggests that the fluid dynamics (air and water) of the ocean environment has lessened some of the physical constraints that operate on land—for example, the similarity between the density of seawater and animal bodies largely reduces the energetic costs of body mass displacement in the ocean compared with land (15). Also, the marine environment is a 3D foraging habitat, a factor that has also been found to decrease the scaling of DR for primates (which use the 3D habitat offered by forest canopies) in comparison with other terrestrial mammals (21). We found no significant phylogenetic differences in the components of movement analyzed here despite the evolutionary histories of these animals spanning millions of years from turtles to polar bears with evolutionary ages of ∼157 million y and ∼150,000 y, respectively.
Our comparative analysis of movement across this diverse group of marine megafauna contributes two key underpinnings to our understanding of their movements. First, internal factors that affect movement are species-specific and independent of the phylogenetic history or traits shared at higher taxonomic or functional groupings (such as family or taxa) and of life-history traits alone, such as breeding strategies. Second, we identified that the key external factor influencing movement of marine megafauna species is their interaction with coastal or open ocean habitats. This finding was corroborated by the dendrogram based on the Kolmogorov–Smirnov distances between the cumulative distribution functions (CDFs) of displacements for each pair of species (Fig. 4). As a consequence, there is a broad diversity of individual movement patterns, ranging from random searching patterns to more directed movement, largely in response to extrinsic forcing (i.e., depending on whether they occur in an offshore or coastal environment). Such differences of scale are consistent with differences in oceanographic processes and the related biophysical coupling (39). We highlight, however, that when available, the internal species-specific factors that may affect movement patterns should be included in future models to further assess how individual movement varies within species.
The study of the different movement behaviors of single species has mostly been framed within the random or optimality paradigms, but when applied in isolation, such theories fail to encompass all of the components associated with movement (7). We propose that a more encompassing framework for understanding animal movement, its connections with habitat, and the species-specific traits that influence it, would define how, where, and why animals move in three sequential levels of analysis. The first level would focus on how animals move by analyzing the characteristics of their displacements, for example, as we have done here using predetermined time windows to understand and describe the observed movement patterns. The second level would focus on quantifying the drivers of movement and specifically on where animals move, for example, by using models to estimate the relative importance of drivers throughout the range of habitats where movement occurs (e.g., open versus coastal oceans or microhabitats). If habitat information is not available, discretizing space into low and high occupancy areas can also be a practical method (40). The final level would focus on why animals move and involve hypothesis testing for specific behaviors as commonly undertaken using the random and the optimality paradigms (12). We expect that a unifying framework of animal movement would consist of the integration of these multiple assessments rather than on the results from specific single assessments completed in isolation. This hierarchical framework encompassing three levels of quantitative exploration to understand movement will provide a strong basis from which to predict potential changes in animal movement associated with forecasted severe environmental changes.
The “common reason for moving” sought by Aristotle and many others since appears to be, at least for the vast group of marine megafauna, largely associated with the contrasting use of the coastal and open ocean habitats. The convergence of movement patterns across marine vertebrates separated by millions of years of evolution and using fundamentally different locomotion modes is remarkable. Given recurring changes in the extent and location of continental shelves over the millennia, the influence of habitat change on the evolution of species should not be underestimated (41–43). Indeed, the importance of understanding paleobiology in the conservation of terrestrial ecosystems was recently identified (44). The importance of habitat shaping the movement patterns of marine megafauna might also be associated with habitat-specific ecological roles of these large species and be key to identifying specific areas of behavioral interest. Our study suggests that efforts to understand marine megafauna movement through analysis of its evolutionary history may yield fewer advances than a focus on understanding the habitats through which animals move (e.g., movement phases in coastal versus open ocean). Such a shift in focus, together with the use of a more encompassing framework, will assist predicting the effects of changes already underway, for example, with the reduction in Arctic shelf areas (45) and predicted sea level rise during the next millennia (46, 47). The great behavioral plasticity of coastal marine vertebrates provides some hope of their higher resilience in a rapidly changing coastal marine environment.
Materials and Methods
Tracking Datasets.
Our dataset spans three decades (1985–2015) and includes a total of 2,557 individuals from 50 marine vertebrate species. Details are given in SI Appendix, SI Materials and Methods.
Probabilistic Analysis of Displacements.
We characterized movement patterns from the time-series of displacements recorded in the spatial trajectories of tagged animals. Displacements were measured as the shortest great circle distance between two locations separated by a predetermined time-window T (e.g., 1 d) along an individual track. Details are given in SI Appendix, SI Materials and Methods.
Assessing Coastal Affinity.
We considered coastal habitats to be those located in emerging and submerged lands within depths of 0–150 m and calculated coastal affinity as the fraction of observed displacements completely occurring within coastal habitats for each individual. Further details are given in SI Appendix, SI Materials and Methods.
BRTs.
We fitted BRT models to the final set of 2,303 individuals across 38 species and the 12 predictor variables (SI Appendix, Tables S2–S4). Modeling details are given in SI Appendix, SI Materials and Methods.
Dendrogram of Movement.
We calculated the Kolmogorov–Smirnov distance between the set of displacements for each species pair when using a time window of 1 d. We then used these distances to produce the dendrogram. Details are given in SI Appendix, SI Materials and Methods.
Supplementary Material
Acknowledgments
We are thankful to I. Jonsen for initial discussions and all involved with the many aspects of fieldwork and data collection; details are included in SI Appendix, Acknowledgments. Workshop funding was granted by the University of Western Australia (UWA) Oceans Institute, the Australian Institute of Marine Science (AIMS), and King Abdullah University of Science and Technology (KAUST). A.M.M.S. was supported by Australian Research Council Grant DE170100841 and an Indian Ocean Ocean Marine Research Centre (UWA, AIMS, Commonwealth of Scientific and Industrial Research Organisation) fellowship. J.P.R., V.M.E., and J.F.G. were supported by Agencia Estatal de Investigación (AEI, Spain) and Fondo Europeo de Desarrollo Regional (FEDER) through project Spatiotemporality in Sociobological Interactions, Models and Methods (SPASIMM) (FIS2016-80067-P AEI/FEDER, European Union), and by research funding from KAUST. J.P.R. was supported by Ministerio de Educación, Cultura y Deporte (Formación de Profesorado Universitario Grant, Spain). D.W.S. was supported by the UK Natural Environment Research Council and Save Our Seas Foundation. N.Q. was supported by Fundação para a Ciência e Tecnologia (Portugal). M.M.C.M. was supported by a Coordenação de Aperfeiçoamento de pessoal de Nível Superior fellowship (Ministry of Education).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The data reported in this paper are archived in digital.csic (dx.doi.org/10.20350/digitalCSIC/8525). Information on how to access the individual datasets is included in SI Appendix.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1716137115/-/DCSupplemental.
References
- 1.Stearns SC. Life-history tactics: A review of the ideas. Q Rev Biol. 1976;51:3–47. doi: 10.1086/409052. [DOI] [PubMed] [Google Scholar]
- 2.Charnov EL. Optimal foraging, the marginal value theorem. Theor Popul Biol. 1976;9:129–136. doi: 10.1016/0040-5809(76)90040-x. [DOI] [PubMed] [Google Scholar]
- 3.Stephens D, Krebs J. Foraging Theory. Princeton Univ Press; Princeton: 1986. [Google Scholar]
- 4.Schmidt-Nielsen K. Locomotion: Energy cost of swimming, flying, and running. Science. 1972;177:222–228. doi: 10.1126/science.177.4045.222. [DOI] [PubMed] [Google Scholar]
- 5.Brown JH, Gillooly JF, Allen AP, Savage VM, West GB. Toward a metabolic theory of ecology. Ecology. 2004;85:1771–1789. [Google Scholar]
- 6.Nussbaum MC. Aristotle’s De Motu Animalium: Text with Translation, Commentary, and Interpretive Essays. Princeton Univ Press; Princeton: 1978. [Google Scholar]
- 7.Nathan R, et al. A movement ecology paradigm for unifying organismal movement research. Proc Natl Acad Sci USA. 2008;105:19052–19059. doi: 10.1073/pnas.0800375105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fauchald P. Foraging in a hierarchical patch system. Am Nat. 1999;153:603–613. [Google Scholar]
- 9.Kareiva P, Odell G. Swarms of predators exhibit preytaxis if individual predators use area-restricted search. Am Nat. 1987;130:233–270. [Google Scholar]
- 10.Bartumeus F, Da Luz MGE, Viswanathan GM, Catalan J. Animal search strategies: A quantitative. Random-walk analysis. Ecology. 2005;86:3078–3087. [Google Scholar]
- 11.Turchin P. Quantitative Analysis of Movement. Sinauer Associates; Sunderland, MA: 1998. [Google Scholar]
- 12.Viswanathan G, da Luz M, Raposo E, Stanley H. The Physics of Foraging: An Introduction to Random Searches and Biological Encounters. Cambridge Univ Press; Cambridge, UK: 2011. [Google Scholar]
- 13.Morales JM, et al. Building the bridge between animal movement and population dynamics. Philos Trans R Soc Lond B Biol Sci. 2010;365:2289–2301. doi: 10.1098/rstb.2010.0082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hays GC, Scott R. Global patterns for upper ceilings on migration distance in sea turtles and comparisons with fish, birds and mammals. Funct Ecol. 2013;27:748–756. [Google Scholar]
- 15.Bale R, Hao M, Bhalla APS, Patankar NA. Energy efficiency and allometry of movement of swimming and flying animals. Proc Natl Acad Sci USA. 2014;111:7517–7521. doi: 10.1073/pnas.1310544111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Singh NJ, Ericsson G. Changing motivations during migration: Linking movement speed to reproductive status in a migratory large mammal. Biol Lett. 2014;10:20140379. doi: 10.1098/rsbl.2014.0379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shaffer SA, Weimerskirch H, Costa DP. Functional significance of sexual dimorphism in Wandering Albatrosses, Diomedea exulans. Funct Ecol. 2001;15:203–210. [Google Scholar]
- 18.Whitlock RE, et al. Direct quantification of energy intake in an apex marine predator suggests physiology is a key driver of migrations. Sci Adv. 2015;1:e1400270. doi: 10.1126/sciadv.1400270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sims DW, et al. Scaling laws of marine predator search behaviour. Nature. 2008;451:1098–1102. doi: 10.1038/nature06518. [DOI] [PubMed] [Google Scholar]
- 20.Garland T. Scaling the ecological cost of transport to body-mass in terrestrial mammals. Am Nat. 1983;121:571–587. [Google Scholar]
- 21.Carbone C, Cowlishaw G, Isaac NJB, Rowcliffe JM. How far do animals go? Determinants of day range in mammals. Am Nat. 2005;165:290–297. doi: 10.1086/426790. [DOI] [PubMed] [Google Scholar]
- 22.Jetz W, Carbone C, Fulford J, Brown JH. The scaling of animal space use. Science. 2004;306:266–268. doi: 10.1126/science.1102138. [DOI] [PubMed] [Google Scholar]
- 23.Donovan ER, Gleeson TT. Scaling the duration of activity relative to body mass results in similar locomotor performance and metabolic costs in lizards. J Exp Biol. 2008;211:3258–3265. doi: 10.1242/jeb.017533. [DOI] [PubMed] [Google Scholar]
- 24.Lidicker WZ, Jr, Stenseth NC. To Disperse or Not to Disperse: Who Does it and Why? Animal Dispersal. Springer; Dordrecht, The Netherlands: 1992. pp. 21–36. [Google Scholar]
- 25.Rubenstein DR, Hobson KA. From birds to butterflies: Animal movement patterns and stable isotopes. Trends Ecol Evol. 2004;19:256–263. doi: 10.1016/j.tree.2004.03.017. [DOI] [PubMed] [Google Scholar]
- 26.Block BA, et al. Tracking apex marine predator movements in a dynamic ocean. Nature. 2011;475:86–90. doi: 10.1038/nature10082. [DOI] [PubMed] [Google Scholar]
- 27.Hindell MA, et al. Circumpolar habitat use in the southern elephant seal: Implications for foraging success and population trajectories. Ecosphere. 2016;7:e01213. [Google Scholar]
- 28.Hussey NE, et al. ECOLOGY. Aquatic animal telemetry: A panoramic window into the underwater world. Science. 2015;348:1255642. doi: 10.1126/science.1255642. [DOI] [PubMed] [Google Scholar]
- 29.Hays GC, et al. Key questions in marine megafauna movement ecology. Trends Ecol Evol. 2016;31:463–475. doi: 10.1016/j.tree.2016.02.015. [DOI] [PubMed] [Google Scholar]
- 30.Barbraud C, Weimerskirch H. Emperor penguins and climate change. Nature. 2001;411:183–186. doi: 10.1038/35075554. [DOI] [PubMed] [Google Scholar]
- 31.Gallagher AJ, Hammerschlag N. Global shark currency: The distribution, frequency, and economic value of shark ecotourism. Curr Issues Tour. 2011;14:797–812. [Google Scholar]
- 32.Queiroz N, et al. Ocean-wide tracking of pelagic sharks reveals extent of overlap with longline fishing hotspots. Proc Natl Acad Sci USA. 2016;113:1582–1587. doi: 10.1073/pnas.1510090113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Elith J, Leathwick JR, Hastie T. A working guide to boosted regression trees. J Anim Ecol. 2008;77:802–813. doi: 10.1111/j.1365-2656.2008.01390.x. [DOI] [PubMed] [Google Scholar]
- 34.Jonsen ID, et al. State-space models for bio-loggers: A methodological road map. Deep Sea Res Part II Top Stud Oceanogr. 2013;88–89:34–46. [Google Scholar]
- 35.Bost CA, et al. The importance of oceanographic fronts to marine birds and mammals of the southern oceans. J Mar Syst. 2009;78:363–376. [Google Scholar]
- 36.Villegas-Amtmann S, Costa DP, Tremblay Y, Salazar S, Aurioles-Gamboa D. Multiple foraging strategies in a marine apex predator, the Galapagos sea lion Zalophus wollebaeki. Mar Ecol Prog Ser. 2008;363:299–309. [Google Scholar]
- 37.Humphries NE, et al. Environmental context explains Lévy and Brownian movement patterns of marine predators. Nature. 2010;465:1066–1069. doi: 10.1038/nature09116. [DOI] [PubMed] [Google Scholar]
- 38.Humphries NE, Weimerskirch H, Queiroz N, Southall EJ, Sims DW. Foraging success of biological Lévy flights recorded in situ. Proc Natl Acad Sci USA. 2012;109:7169–7174. doi: 10.1073/pnas.1121201109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Steele JH, Henderson EW. Coupling between physical and biological scales. Philos T Roy Soc B. 1994;343:5–9. [Google Scholar]
- 40.Rodríguez JP, et al. Big data analyses reveal patterns and drivers of the movements of southern elephant seals. Sci Rep. 2017;7:112. doi: 10.1038/s41598-017-00165-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Harley CDG, et al. The impacts of climate change in coastal marine systems. Ecol Lett. 2006;9:228–241. doi: 10.1111/j.1461-0248.2005.00871.x. [DOI] [PubMed] [Google Scholar]
- 42.Schipper J, et al. The status of the world’s land and marine mammals: Diversity, threat, and knowledge. Science. 2008;322:225–230. doi: 10.1126/science.1165115. [DOI] [PubMed] [Google Scholar]
- 43.Halpern BS, et al. A global map of human impact on marine ecosystems. Science. 2008;319:948–952. doi: 10.1126/science.1149345. [DOI] [PubMed] [Google Scholar]
- 44.Barnosky AD, et al. Merging paleobiology with conservation biology to guide the future of terrestrial ecosystems. Science. 2017;355:eaah4787. doi: 10.1126/science.aah4787. [DOI] [PubMed] [Google Scholar]
- 45.Post E, et al. Ecological consequences of sea-ice decline. Science. 2013;341:519–524. doi: 10.1126/science.1235225. [DOI] [PubMed] [Google Scholar]
- 46.Nicholls RJ, Cazenave A. Sea-level rise and its impact on coastal zones. Science. 2010;328:1517–1520. doi: 10.1126/science.1185782. [DOI] [PubMed] [Google Scholar]
- 47.Dutton A, et al. SEA-LEVEL RISE. Sea-level rise due to polar ice-sheet mass loss during past warm periods. Science. 2015;349:aaa4019. doi: 10.1126/science.aaa4019. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.