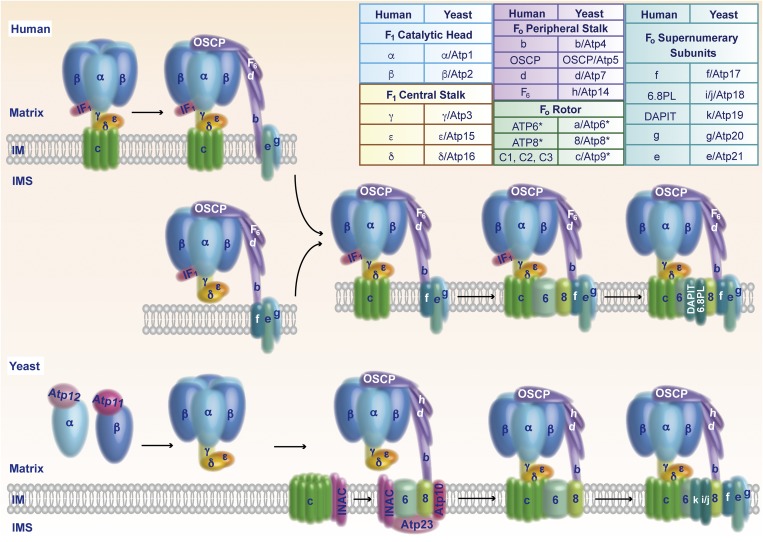
Fig. 1.
Assembly pathways of the mitochondrial ATP synthase. (Upper) Assembly of human ATP synthase. The free F1 domain or an F1–c-ring intermediate binds to the peripheral stalk. The supernumerary subunits e, g, and f associate and promote the insertion of ATP6 and ATP8. Addition of 6.8PL and DAPIT stabilizes the inserted ATP6/ATP8, leading to formation of the proton-conducting channel between ATP6 and the c-ring. The inhibitory protein IF1 that blocks ATP hydrolysis of uncoupled ATP synthase is released. (Lower) Assembly of yeast ATP synthase. The chaperones Atp11 and Atp12 promote formation of the F1 domain. The INAC binds to the c-ring, as well as to an assembly intermediate that contains Atp6, Atp8, the peripheral stalk, the F1 domain, and the maturation factors Atp10 and Atp23. The INAC thus supports proper association of the c-ring with Atp6 to allow formation of the proton-conducting channel. Subsequently, the supernumerary subunits are added to promote dimerization of the enzyme. (Inset) Structural subunits of human and yeast ATP synthase. Asterisks mark mitochondrially encoded subunits. IM, inner mitochondrial membrane; IMS, intermembrane space; OSCP, oligomycin sensitivity-conferring protein.