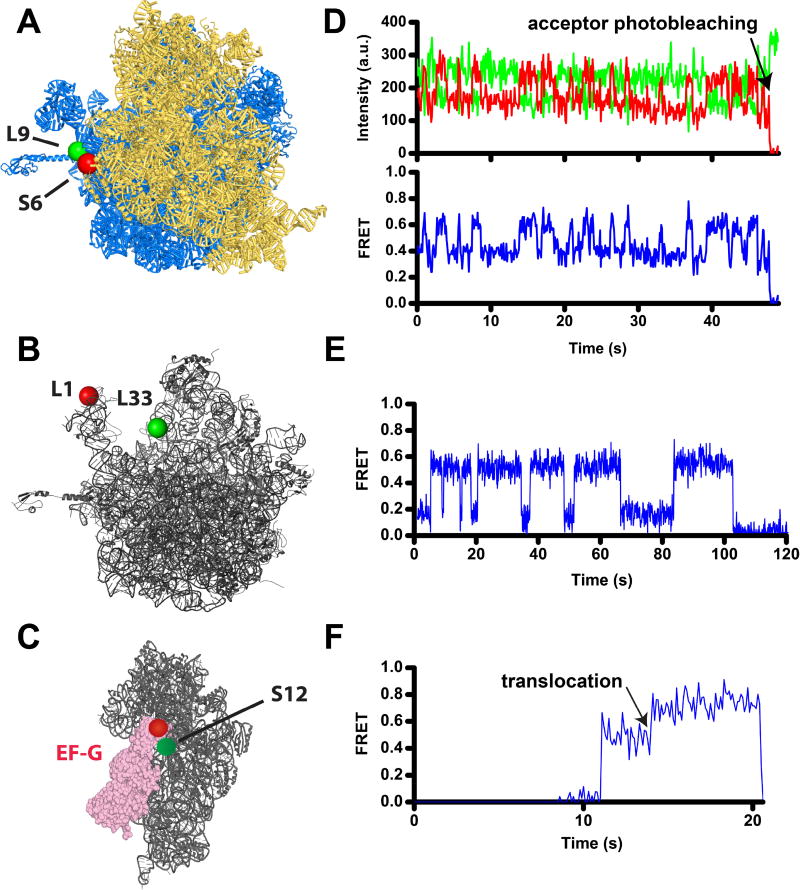
Figure 1. Following translocation and ribosome dynamics using smFRET.
A) Intersubinit rotation is followed by FRET between a donor (Cy3, green) and an acceptor (Cy5, red) attached to ribosomal proteins bL9 and bS6 on the large (blue) and small (gold) ribosomal subunits, respectively [80, 106, 122]. View from the solvent side of the small subunit of the 70S ribosome from E. coli in the nonrotated conformation (PDBID 4V9D [123]). In this view, the formation of the rotated conformation of the ribosome results in counterclockwise rotation of the small subunit. B) The movement of the 50S L1 stalk of the large subunit (grey) is followed by FRET between Cy5 (red) and Cy3 (green) attached to proteins uL1 and bL33, respectively [84, 122] (PDBID 4V51 [124]). C) Docking of domain IV of EF-G (pink) to the A site of the small subunit (grey) during translocation is followed by FRET between Cy3 (red) and Cy5 (red) attached to EF-G and protein uS12, respectively [28]. The small subunit of the posttranslocated ribosome is viewed from the subunit interface (PDBID 4V5F [125]). D–F) smFRET traces show fluorescence intensities for Cy3 (green) and Cy5 (red) (D), and FRET efficiency (blue, D–F). D) S6/L9 smFRET trace shows spontaneous fluctuations of a ribosome, which contains deacylated tRNAfMet in the P site, between the rotated and nonrotated conformations that correspond to 0.4 and 0.6 FRET states. E) L1/L33 smFRET trace shows fluctuations of the L1 stalk in a ribosome, which contains deacylated tRNAfMet in the P site, between the open and closed conformations that correspond to 0.2 and 0.5 FRET states. F) S12-EF-G smFRET trace shows a transition from 0.55 to 0.8 FRET that corresponds to the movement of domain IV toward the A site of the small subunit during translocation of tRNA/mRNA.