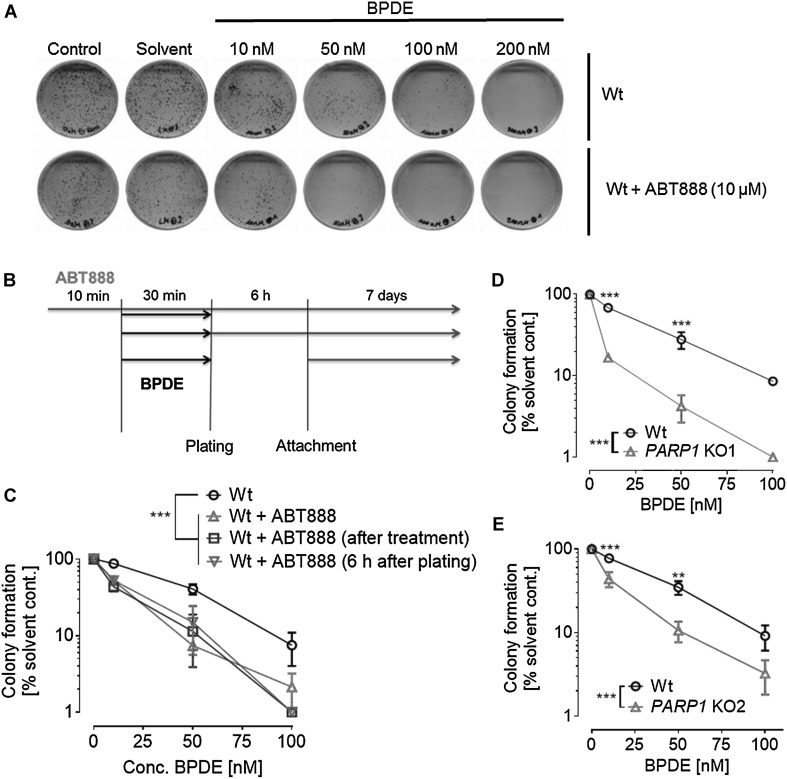
Fig. 4.
PARP inhibition or genetic PARP1 ablation potentiates BPDE’s long-term toxicity. HeLa Wt or PARP1 knockout (KO1/KO2) cells were treated with increasing concentrations of BPDE for 30 min and clonogenic survival assays were performed by incubating cells for 7-day posttreatment. a Representative cell culture dishes showing results from a clonogenic survival assay performed with cells treated with BPDE in concentrations as indicated. In all experiments, no colony formation was observed upon 200-µM BPDE treatment. b Scheme showing the different treatment schedules to test for the importance of timing of PARP inhibition and BPDE exposure. Three different treatment schedules were tested. c Quantification of clonogenic survival assays described in (b). Red: ABT888 was present before, during, and after BPDE treatment. Blue: ABT888 was added directly after the treatment. Green: ABT888 was added 6 h after BPDE exposure. No major influence on BPDE’s toxicity could be observed when altering the timing of PARP inhibition. All three ABT888 approaches showed significantly less colonies than the ABT888-untreated one while displaying only minor differences in-between the different ABT888 treatment schedules. d, e Similar to pharmacological PARP inhibition, genetic PARP1 ablation also potentiated BPDE’s induced long-term cytotoxicity. Data represent means ± SEM of ≥ 3 independent experiments and each performed in technical triplicates, normalized to solvent control. For control assays, see Suppl. Figure 5. Statistical evaluation was performed using two-way ANOVA analysis followed by Sidak’s multiple comparison testing. *p < 0.05, **p < 0.01, ***p < 0.001. (Color figure online)