Abstract
OBJECTIVES
Frailty, a critical determinant of health outcomes, is most commonly assessed in patients with cirrhosis by general clinician assessment that is limited by its subjectivity. We aimed to compare the objective Liver Frailty Index (LFI), consisting of three performance-based tests (grip, chair stands, balance), with a subjective hepatologist assessment.
METHODS
Outpatients with cirrhosis awaiting liver transplantation (LT) underwent: (1) objective measurement using the LFI and (2) subjective clinician assessment. Spearman’s correlation assessed associations between the LFI and clinician assessment; Cox regression with waitlist mortality (death/delisting for sickness); discriminative ability with Concordance(C) statistics. The net reclassification index evaluated the percentage of patients correctly reclassified by adding the LFI to the clinician assessment.
RESULTS
Of the 529 patients with cirrhosis, median LFI was 3.8 (range 1.0–7.0) and clinician assessment was 3 (range 0–5). Correlation between LFI and the clinician assessment was modest (ρ=0.38) with high variability by hepatologist (ρ=0.26–0.70). At a median of 11 months, 102 (19%) died/were delisted. Both the LFI (hazard ratio (HR) 2.2, 95% confidence interval (CI) 1.7–2.9) and clinician assessment (HR 1.6, 95% CI 1.3–1.9) were associated with adjusted waitlist mortality risk (P<0.01). The addition of the LFI to the clinician assessment significantly improved mortality prediction over the clinician assessment alone (0.74 vs. 0.68; P=0.02). Compared with the clinician assessment alone, the addition of the LFI correctly reclassified 34% (95% CI 8–53%) of patients to their correct survival status.
CONCLUSION
The subjective clinician assessment can predict waitlist mortality in patients with cirrhosis but is subjective and variable by hepatologist. The addition of the LFI to the subjective clinician assessment significantly improved mortality risk prediction, reclassifying 34% of patients. Our data strongly support the incorporation of the objective LFI to anchor our assessments of patients with cirrhosis to enhance our decision-making.
INTRODUCTION
One of the most important “tools” that a clinician uses for medical decision-making is his or her overall appraisal of a patient’s health. For a clinician caring for a patient with cirrhosis, this holistic judgment of health may incorporate objective data, including vital signs, the Model for End-Stage Liver Disease (MELDNa) score, and results from other procedures (e.g., abdominal imaging, transthoracic echocardiogram). At the heart of this appraisal is the “eyeball test”, which may take into account the patient’s mobility, visual estimations of muscle bulk, and mental approximations of the patient’s ability for physical exertion.
This global clinician assessment is critical to hepatology and liver transplantation because it seamlessly combines objective and subjective information for prognostication—both before and after liver transplantation—in ways that cannot fully be accounted for by any disease or physiological measure, including MELDNa, Child Pugh score, or quantification of skeletal muscle mass. In fact, we have previously demonstrated that hepatologists can reasonably predict mortality in patients with cirrhosis awaiting liver transplantation, independent of liver disease severity (1). However, because it is rooted in the “eyeball test”—which currently lacks any standardized, objective measurement—the subjective clinician assessment may result in high variability in clinical decision-making. This is particularly problematic for patients awaiting liver transplantation, where decisions about listing—or de-listing—are matters of life and death.
We have demonstrated that objective instruments to operationalize the geriatric concept of “frailty”, a distinct biological syndrome of decreased physiological reserve (2), have construct validity for the “eyeball test” (3,4) What is not known, however, is whether an objective measure of frailty can improve subjective clinician assessment with respect to mortality prediction. If so, such an objective measure could increase equity and transparency in transplant decision-making.
METHODS
Patients and their clinicians
We used data from the Functional Assessment in Liver Transplantation (FrAILT) Study from March 2012 to July 2016; the full FrAILT Study protocol has been published in full (3). Briefly, patients with cirrhosis who were actively listed for liver transplantation at the University of California, San Francisco and seen as outpatients were eligible for enrollment. Excluded were patients with severe hepatic encephalopathy (n=14), as defined by the time to complete a Numbers Connection Test (5) of >120 s, or those who did not speak English, as these reasons may impair the patient’s ability to provide informed consent and complete tests of physical function. For the purposes of this specific study, patients listed for liver transplantation with exception points for hepatocellular carcinoma were also excluded, as need for liver transplantation differs for these patients from those listed for liver transplantation with cirrhosis and portal hypertensive complications. Lastly, we excluded patients (n=52) who were not seen by a transplant hepatologist on the same day as their objective frailty measurement (e.g., they were seen by a nurse practitioner or physician’s assistant). No patients in our cohort had purely structural reasons that would impair their ability to complete performance-based testing of physical function (e.g., lower extremity amputation, paraplegia).
In the UCSF Liver Transplant Program, each waitlist candidate is cared for by a transplant hepatologist, who manages the candidate from evaluation to transplant. Nine transplant hepatologists at UCSF participated in this study. The hepatologists were categorized as “senior” if, by 2016, they had >4 years of clinical practice or “junior” if ≤4 years of clinical practice.
Study procedures
All patients underwent objective measurement of frailty using:
Grip strength (2): the average of three trials, measured in the subject’s dominant hand using a hand dynamometer;
Timed chair stands (6): measured as the number of seconds it takes to do five chair stands with the subject’s arms folded across the chest;
Balance testing (6): measured as the number of seconds that the subject can balance in three positions (feet placed side-to-side, semitandem, and tandem) for a maximum of 10 s each.
These three tests were administered by trained study personnel. With these three individual tests of frailty, the Liver Frailty Index was calculated using the following equation (3) (calculator available at: http://liverfrailtyindex.ucsf.edu):
Based on data collected from 42 patients with cirrhosis who underwent frailty measurement using the Liver Frailty Index on the same day, test–retest reliability (7) was 0.88 within 0.5 units of the Liver Frailty Index and 0.93 within Liver Frailty Index categories of robust, prefrail, and robust.
On the same day as the clinic visit, the patient’s hepatologist who provided outpatient care to the patient was asked to subjectively rate his or her patient’s health (“subjective clinician assessment”) using the following question:
“We are interested in your general impression about your patient’s overall health, as compared with other patients with underlying liver disease. How would you rate this patient’s overall health today? Excellent (0), very good (1), good (2), fair (3), poor (4), or very poor (5)”.
The hepatologists were blinded to the frailty measurements at the time of answering this question. We have previously demonstrated that this subjective clinician assessment can identify liver transplant candidates at high risk for waitlist mortality (1).
Data regarding demographics were extracted from the clinic visit note from the same day as the objective frailty measurement. Patients were considered to have a diagnosis of hypertension or diabetes if this diagnosis was reported in their electronic health record or they were taking medications for either of these diseases (as advancing portal hypertension may affect the manifestation of hypertension or diabetes). Ascites was ascertained from the hepatologists’ recorded physical examination or the management plan and graded as none, mild/moderate, or refractory. Hepatic encephalopathy was determined from the time to complete the Numbers Connection Test (5) performed at the time of the frailty measurement and categorized as none/minimal (<60 s) or moderate/severe (≥60 s).
Statistical analysis
Spearman’s rank correlation coefficient and linear regression quantified associations between the Liver Frailty Index, the subjective clinician assessment, and clinical characteristics. Correlation coefficients were compared with bias-corrected bootstrapping (8). Bootstrapping was used because comparing the correlation coefficients between two models fit to the same data set is a non-standard analysis and the distribution of correlation coefficients can be highly non-normal.
The primary outcome was waitlist mortality, which we defined as a combined outcome of death or delisting for being too sick for liver transplantation. Patients who were removed for reasons other than being too sick (i.e., for social reasons) were censored on the day of their removal from the waitlist. Patients who underwent living donor liver transplantation were also censored on the day of their liver transplantation. Associations between the Liver Frailty Index or the subjective clinician assessment with waitlist mortality were evaluated using Cox regression. Z-statistics were also presented for comparison of the two predictors (as they are scaled differently); the higher the z -statistic, the greater the sensitivity of the predictor. All variables associated with waitlist mortality with a P-value of 0.1 in univariable analysis were evaluated for inclusion in the final multivariable model. Backwards stepwise regression was then performed to derive the final multivariable model, which included only variables associated with a P-value <0.05.
The discriminative abilities for predicting waitlist mortality (using Cox regression) of the Liver Frailty Index, the subjective clinician assessment, or the two combined were assessed with Concordance (C) statistics using Cox regression and compared with bias-corrected bootstrapping (for the same reason we used bootstrapping for the correlation coefficients) (8). Although Cox regression can sometimes lead to overestimation of risk in multistate models (9), estimation of C-statistics is not available with competing risks regression in Stata. Therefore, we also provided estimates from the multivariable models using competing risks regression (10), which demonstrates that use of competing risks regression does not substantially change the qualitative interpretation that both the Liver Frailty Index and the subjective clinician assessment were significantly associated with waitlist mortality. Patients who underwent living donor liver transplantation were censored at the time of liver transplantation for this analysis.
To evaluate the incremental value of the Liver Frailty Index to the subjective clinician assessment on improving prediction of waitlist mortality, we compared the proportion of patients whose risk of waitlist mortality, estimated from the Cox model (as net reclassification methodology is not available using competing risks regression), was correctly reclassified using the subjective clinician assessment plus the Liver Frailty Index vs. the subjective clinician assessment alone. This comparison was assessed using the continuous net reclassification index (“INCRISK” program in Stata) (11).
Statistical analyses were performed using Stata (v14, Stata, College Station, TX). The Institutional Review Board at the University of California, San Francisco approved this study.
RESULTS
Characteristics of the patient population
A total of 529 patients were included in this study. Baseline characteristics of the cohort are shown in Table 1. To briefly summarize, median age was 58 years, 42% were female, 57% were non-Hispanic White, and median body mass index was 28 kg/m2. Twenty-eight percent had chronic hepatitis C as their primary etiology of liver disease, 39% carried a diagnosis of hypertension, and 28% diabetes. In this outpatient cohort, median MELDNa was 18 and albumin was 3.0 g/dl. The proportion with Child Class A, B, and C was 15, 63, and 22%, respectively. At a median follow-up time of 11 months, 106 (20%) experienced the primary outcome of death/delisting for being too sick for liver transplantation, 229 (42%) underwent deceased donor liver transplantation, and 46 (9%) underwent living donor liver transplantation (and were censored at the time of their transplant for the analyses).
Table 1.
Characteristics of the 529 patients with cirrhosis enrolled in this study
| Characteristics | n=529 |
|---|---|
| Age, years | 58 (50–63) |
| Female | 220 (42%) |
| Race | |
| Non-Hispanic White | 307 (58%) |
| Black | 17 (3%) |
| Hispanic White | 133 (25%) |
| Asian/Pacific Islander | 24 (5%) |
| Other | 48 (9%) |
| Body mass index, kg/m2 | 28 (25–33) |
| Etiology of liver disease | |
| Chronic hepatitis C | 203 (38%) |
| Alcohol | 122 (23%) |
| Non-alcoholic steatohepatitis | 72 (14%) |
| Autoimmune/cholestatic | 77 (15%) |
| Other | 55 (10%) |
| Hypertension | 204 (39%) |
| Diabetes | 145 (27%) |
| MELDNa | 18 (15–23) |
| Total bilirubin, mg/dl | 2.6 (1.7–4.2) |
| Creatinine, mg/dla | 0.91 (0.72–1.20) |
| International normalized ratio for prothrombin time | 1.4 (1.3–1.6) |
| Sodium, mEq/l | 136 (133–139) |
| Albumin, g/dl | 3.0 (2.6–3.4) |
| Dialysis | 23 (4%) |
| Ascites | |
| Mild/moderate | 143 (27%) |
| Refractory | 35 (7%) |
| Hepatic encephalopathy | 99 (19%) |
| Dialysis | 23 (4%) |
| Child Pugh Class | |
| A | 78 (15%) |
| B | 335 (64%) |
| C | 108 (21%) |
| Follow-up time, months | 11 (4–21) |
| Outcome | |
| Waiting | 224 (42%) |
| Death/delisted for being too sick for transplant | 102 (19%) |
| Deceased donor liver transplant | 131 (25%) |
| Other | 72 (14%) |
MELDNa, Model for End-Stage Liver Disease.
Values are median (interquartile range) or n (%).
Among those who were not on dialysis.
Characteristics of the clinicians
Of the nine board-certified transplant hepatologists who participated in this study, five were women and four were men. The median number of years in practice as a hepatologist (as of 2016) was 4 (interquartile range 3–9; range 3–21).
Frailty assessments by the Liver Frailty Index and subjective clinician assessments
Median (interquartile range) Liver Frailty Index was 3.8 (3.4–4.3), and the full range of scores was 1.0–7.0 (with a higher score indicating that the patient was more frail). A total of eight trained personnel administered the Liver Frailty Index to patients over the course of the study period; there was no statistically significant difference in the median Liver Frailty Index scores by assessor (P=0.28). Median (interquartile range) subjective clinician assessment score was 2 (1–3) with a full range of 0–5 (with a higher score indicating that the patient was more frail). Both the Liver Frailty Index and the subjective clinician assessment were positively associated (i.e., indicating a greater degree of frailty) with diagnoses of non-alcoholic steatohepatitis, the presence of ascites or hepatic encephalopathy, and Child Pugh Class C and negatively associated with increasing albumin and longer follow-up time (Table 2). The subjective clinician assessment was significantly associated with patient sex (female patients were rated 0.26 points more frail than men; P=0.01) but the Liver Frailty Index was not (Table 2). Conversely, the Liver Frailty Index was associated with advancing age (each 1 year increase was associated with a 0.01 increase in the Liver Frailty Index; P<0.001) and a diagnosis of diabetes (diabetic patients were 0.25 points more frail than non-diabetic patients; P=0.001) while the subjective clinician assessment was not. Neither was associated with race nor a diagnosis of hypertension (Table 2).
Table 2.
Associations between patient characteristics and the Liver Frailty Index or subjective clinician assessment using linear regression
| Patient characteristic | Liver Frailty Index | Subjective clinician assessment | ||
|---|---|---|---|---|
|
|
|
|||
| Coefficient (95% CI) | P-value | Coefficient (95% CI) | P-value | |
| Age, per year | 0.01 (0.01 to 0.02) | <0.001 | 0.01 (−0.003 to 0.02) | 0.14 |
|
| ||||
| Female sex | 0.05 (−0.09 to 0.19) | 0.50 | 0.26 (0.04 to 0.48) | 0.02 |
|
| ||||
| Race | ||||
|
| ||||
| Non-Hispanic White | Ref. | Ref. | ||
|
| ||||
| Black | −0.03 (−0.43 to 0.36) | 0.87 | −0.35 (−0.95 to 0.26) | 0.26 |
|
| ||||
| Hispanic White | 0.12 (−0.04 to 0.28) | 0.15 | 0.17 (−0.08 to 0.43) | 0.18 |
|
| ||||
| Asian/Pacific Islander | −0.01 (−0.35 to 0.32) | 0.93 | −0.21 (−0.74 to 0.31) | 0.42 |
|
| ||||
| Other | 0.09 (−0.16 to 0.34) | 0.47 | 0.11 (−0.27 to 0.49) | 0.56 |
|
| ||||
| Body mass index, per unit kg/m2 | 0.009 (−0.003 to 0.02) | 0.13 | 0.003 (−0.02 to 0.02) | 0.77 |
|
| ||||
| Non-alcoholic steatohepatitisa | 0.36 (0.15 to 0.58) | 0.001 | 0.48 (0.15 to 0.82) | 0.005 |
|
| ||||
| Hypertension | 0.10 (−0.04 to 0.24) | 0.15 | 0.08 (−0.14 to 0.30) | 0.48 |
|
| ||||
| Diabetes | 0.25 (0.10 to 0.41) | 0.001 | 0.18 (−0.05 to 0.42) | 0.13 |
|
| ||||
| MELDNa, per point | 0.04 (0.03 to 0.06) | <0.001 | 0.09 (0.08 to 0.11) | <0.001 |
|
| ||||
| Albumin, per g/dl | −0.20 (−0.32 to −0.09) | 0.001 | −0.56 (−0.73 to −0.39) | <0.001 |
|
| ||||
| Ascites | ||||
|
| ||||
| None | Ref. | Ref. | ||
|
| ||||
| Mild/moderate | 0.35 (0.20 to 0.51) | <0.001 | 0.57 (0.33 to 0.80) | <0.001 |
|
| ||||
| Refractory | 0.48 (0.20 to 0.75) | 0.001 | 0.91 (0.49 to 1.33) | <0.001 |
|
| ||||
| Hepatic encephalopathy | 0.48 (0.31 to 0.65) | <0.001 | 0.46 (0.19 to 0.73) | 0.001 |
|
| ||||
| Child Pugh Class | ||||
|
| ||||
| A | Ref. | Ref. | ||
|
| ||||
| B | 0.12 (−0.07 to 0.32) | 0.22 | 0.82 (0.54 to 1.11) | <0.001 |
|
| ||||
| C | 0.48 (0.25 to 0.72) | <0.001 | 1.60 (1.26 to 1.93) | <0.001 |
|
| ||||
| Follow-up time, months | −0.02 (−0.02 to −0.01) | <0.001 | −0.03 (−0.03 to −0.02) | <0.001 |
CI, confidence interval; MELDNa, Model for End-Stage Liver Disease.
Of all the liver disease etiologies, only the association between non-alcoholic steatohepatitis and the Liver Frailty Index/subjective clinician assessment was statistically significant.
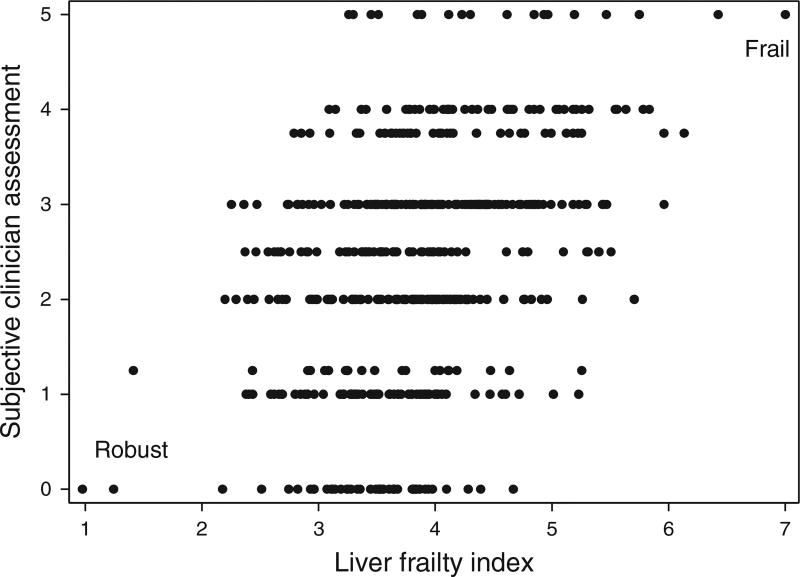
Spearman’s correlation coefficient between the Liver Frailty Index and the subjective clinician assessments was 0.38 (P<0.001; Figure 1). This correlation varied by hepatologist, ranging from ρ=0.29 to ρ=0.70. The correlation between the Liver Frailty Index and the subjective clinician assessments for senior hepatologists (>4 years in practice) was 0.35 (P<0.001) and for junior hepatologists (≤4 years in practice) was 0.48 (P<0.001) (P=0.07 for the comparison between senior and junior hepatologists). There was modest correlation between the Liver Frailty Index and the subjective clinician assessments among female hepatologists (ρ=0.47; P<0.001) and among male hepatologists (ρ=0.34; P<0.001) (P=0.05).
Figure 1.
Correlation between the Liver Frailty Index and subjective clinician assessment scores (Spearman’s ρ=0.38; P<0.001).
Prognostic value of the Liver Frailty Index and subjective clinician assessments
At a median follow-up of 11 months, 102 (19%) died or were delisted. In univariable analysis, each unit increase in the Liver Frailty Index (i.e., indicating a greater degree of frailty) was significantly associated with waitlist mortality in using both Cox regression (hazard ratio (HR) 2.9; 95% confidence interval (CI) 2.2–3.7; P<0.001) and competing risks regression (HR 2.3; 95% CI 1.8–3.0; P<0.001). A significant association was also found for the subjective clinician assessment in both Cox regression (HR 1.9; 95% CI 1.6–2.3; P<0.001) and competing risks regression (HR 1.6; 95% CI 1.3–1.9; P<0.001). In separate multivariable analyses with demographic and clinical parameters, both the Liver Frailty Index and the subjective clinician assessment remained significantly associated with waitlist mortality, regardless of regression methodology (Table 3).
Table 3.
Multivariable models to assess associations with waitlist mortality using Cox and competing risks (with deceased donor liver transplant as the competing risk) regressions for: (A) the Liver Frailty Index and (B) the subjective clinician assessmenta
| Cox regression | Competing risks regression | |||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Hazard ratio (95% CI) | z | P-value | Hazard ratio (95% CI) | z | P-value | |
| (A) | ||||||
|
| ||||||
| Liver Frailty Index, per point | 2.21 (1.68–2.91) | 5.6 | <0.001 | 1.99 (1.50–2.64) | 4.8 | <0.001 |
|
| ||||||
| Age, per year | — | — | — | 1.03 (1.01–1.05) | 2.5 | 0.01 |
|
| ||||||
| MELDNa, per point | 1.11 (1.06–1.15) | 4.8 | <0.001 | 1.05 (1.01–1.10) | 2.3 | 0.02 |
|
| ||||||
| Hepatic encephalopathy | 1.64 (1.05–2.55) | 2.2 | 0.03 | — | — | — |
|
| ||||||
| (B) | ||||||
|
| ||||||
| Subjective clinician assessment | 1.59 (1.30–1.94) | 4.6 | <0.001 | 1.39 (1.14–1.69) | 3.3 | 0.001 |
|
| ||||||
| Age, per year | — | — | — | 1.03 (1.00–1.05) | 2.2 | 0.03 |
|
| ||||||
| MELDNa, per point | 1.11 (1.06–1.16) | 4.9 | <0.001 | 1.06 (1.01–1.10) | 2.6 | 0.01 |
|
| ||||||
| Hepatic encephalopathy | 1.91 (1.24–2.96) | 2.9 | 0.004 | 1.67 (1.08–2.58) | 2.3 | 0.02 |
CI, confidence interval; MELDNa, Model for End-Stage Liver Disease.
Variables evaluated for inclusion in the multivariable models were: age, sex, body mass index, etiology of liver disease, hypertension, diabetes, MELDNa, albumin, ascites, hepatic encephalopathy (on the day of frailty testing), and Child Pugh Class. Only variables associated with a P-value <0.1 were evaluated in the final multivariable model. Only variables associated with a P-value <0.01 were retained in the final models.
The ability of the Liver Frailty Index and the subjective clinician assessment to correctly rank patients according to their risk of death (C-statistic) was 0.71 and 0.68, respectively (P=0.41 for the comparison; Table 4). However, the addition of the Liver Frailty Index to the subjective clinician assessment significantly improved waitlist mortality prediction over the subjective clinician assessment alone (0.74 vs. 0.68; P<0.02) but addition of the subjective clinician assessment did not significantly improve mortality prediction over the Liver Frailty Index alone (0.74 vs. 0.71; P=0.31; Table 4). Compared with the subjective clinician assessment alone, the addition of the Liver Frailty Index correctly reclassified 17% (95% CI 1–30%) of deaths/delisting and 17% (95% CI 4–26%) of non-deaths/non-delistings for a total net reclassification index of 34% (95% CI 9–53%).
Table 4.
Concordance (C) statistics for the subjective clinician assessment, Liver Frailty Index, and both predictors together, with comparison P-values
| A | B | C | P-value A vs. B | P-value B vs. C | P-value A vs. C | |
|---|---|---|---|---|---|---|
|
|
|
|
||||
| Subjective clinician assessment |
Liver Frailty Index | Subjective clinician assessment+Liver Frailty Index |
||||
| C-statistic | 0.68 | 0.71 | 0.74 | 0.41 | 0.31 | 0.02 |
DISCUSSION
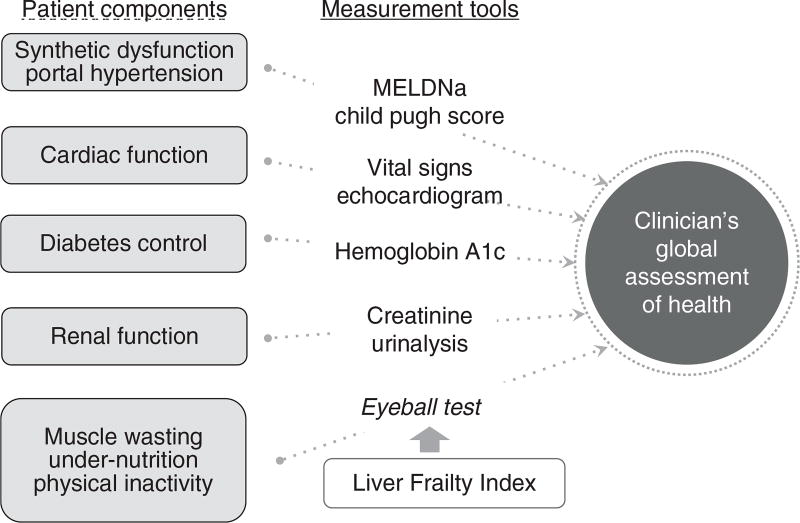
There is no more quintessential display of the art of medicine than the clinician’s global assessment of health. In this holistic assessment, a clinician synthesizes the components of a patient’s history and physical examination and objective laboratory and radiographic data with his or her “eyeball test”—an observation of the patient’s relation to the immediate environment (i.e., how a patient moves from the chair to the exam table)—into a single, simple appraisal that is made at the bedside almost instantly (Figure 2). Multiple studies have demonstrated that clinicians can predict mortality in hospitalized patients with relative accuracy—and oftentimes as well as traditional, quantitative risk indices (12–17).
Figure 2.
A conceptual model of some of the patient components that clinicians incorporate into their global assessment of a patient’s health and the tools that they use to inform this holistic assessment. The objective Liver Frailty Index should be used to inform the eyeball test to improve objectivity and accuracy of the subjective clinician assessment. MELDNa, Model for End-Stage Liver Disease. A full color version of this figure is available at the American Journal of Gastroenterology journal online.
But there are problems with relying too heavily on the eyeball test in this global appraisal for clinical decision-making. It is subjective and may be influenced by factors that may have little to do with a patient’s prognosis, such as the time of day the patient was seen, quality of clothing that the patient is wearing, or even factors specific to the clinician himself (or herself). Conversely, the eyeball test may miss subtle signs that are undetectable by the naked eye but strongly associated with prognosis, such as slight slowing of gait speed (18) or progressive muscle wasting (19,20). The incorporation or omission of these factors may exert greater influence on the eyeball test in the outpatient setting, where patients are in a less dynamic disease state and where subtle signs have a much larger role in a patient’s prognosis. Its subjectivity leaves it open to variability from one provider to another. Objective tools of the factors that clinicians incorporate into the global appraisals of their patients’ health—and that have prognostic value—are greatly needed.
In this study, we evaluated an objective test of physical frailty, the Liver Frailty Index (which we have previously demonstrated has strong prognostic value over the MELDNa alone (3)), in the context of understanding and improving the clinician assessment of global health. The great advantage of measuring frailty in this population is the ability to account for the muscle atrophy and dysfunction that occurs as a result of not only the cirrhosis itself but from advancing age and comorbidities (21), both of which are increasing in prevalence in the liver transplant population. Our selection of the Liver Frailty Index for this comparison—rather than other tests of frailty or physical function that also have prognostic value in patients with cirrhosis—was deliberate. We have previously demonstrated that this index has strong construct validity for frailty (22) and also captures factors such as diabetes and dialysis dependence (unpublished data). Unlike instruments that incorporate subjective, patient-reported domains, such as the Fried Frailty Instrument (4), the Braden Scale (23), or Activities of Daily Living scale (23,24), the Liver Frailty Index consists of only performance-based tests that are objective. In contrast to the performance-based Short Physical Performance Battery (6,25), the Liver Frailty Index is continuous, making it better able to detect longitudinal changes in physical function. Finally, the Liver Frailty Index has a strong advantage over cardiopulmonary exercise testing (26) or the 6-min walk test (27) in that it can be performed at the bedside in approximately 2 min, about as long as it takes to measure routine vital signs in the clinic. We have also previously demonstrated that changes over time in the components included in the Liver Frailty Index (grip strength, chair stands, and balance) are associated with waitlist mortality, independent of baseline physical function (18). We observed that the correlation between the Liver Frailty Index and the subjective clinician assessment was modest, suggesting some overlap in the two but leaving room for one test to inform the other. Indeed, we found that the addition of the Liver Frailty Index to the subjective clinician assessment significantly improved predictions of waitlist mortality in this population.
Our choice of the subjective clinician assessment tool that we used for this study was equally deliberate. As our objective was to improve upon the clinician assessment—rather than evaluate the clinician’s ability to accurately assess frailty or functional status—we sought a question that captured the clinician’s gut sense of how the patient was doing overall, as in, “This patient eyeballs well.” To accomplish this, we used a standard health assessment question derived from the National Health Interview Study (28). We felt that this better captured overall health status, rather than the Clinical Frailty Scale or Karnofsky Performance status, which are subjective tools to assess frailty and functional status and have previously been shown to predict clinically relevant outcomes in cirrhotic patients (in studies published after initiation of the FrAILT Study) (29,30). Importantly, we have previously demonstrated that this subjective clinician assessment tool can identify liver transplant candidates at high risk for waitlist mortality (1), supporting its use in this study. Although we have not evaluated the reproducibility of the subjective clinician assessment, any weaknesses in the reproducibility of this tool would only strengthen our overall study objective to standardize this subjective assessment.
We acknowledge several limitations to this study. Given that our study population included only patients with cirrhosis listed for liver transplantation, our results may not be generalizable to the entire population of patients with cirrhosis as a whole. We have not yet evaluated the reproducibility of the Liver Frailty Index; however, median Liver Frailty Index scores were similar by assessor. In addition, reproducibility of the individual components is high in patients without liver disease, with test–retest reliability of 0.85 for grip strength (31), 0.73–0.78 for chair stands (32), and 0.55–0.75 for balance (32), so we anticipate similarly good reproducibility in our patient population (this study is currently underway). Second, owing to limitations of available statistical programs, we were only able to evaluate model discrimination from Cox regression, which may overestimate risk in multi-state models (9). However, evaluation of the associations with waitlist mortality using Cox regression and competing risks analysis (Table 3) demonstrated consistently strong association between the Liver Frailty Index or the subjective clinician assessment with waitlist mortality regardless of the regression method employed. Furthermore, the method of regression should not change our key conclusion that the combination of both the Liver Frailty Index and the subjective clinician assessment improves prediction of waitlist mortality over the subjective clinician assessment alone. Lastly, while tests of frailty to prognosticate among hospitalized cirrhotic patients are greatly needed, our study is not generalizable to the inpatient setting. However, our ultimate goal is to use the information obtained from the Liver Frailty Index to identify patients in greatest need of prehabilitation and identifying these patients as outpatients—when there is sufficient time to prehabilitate for liver transplantation—is critical.
Despite these limitations, our observations have important and practical implications. Although subjective assessment of patients by their clinicians is an indispensable component of clinical practice, this assessment could be improved by objective data about a patient’s physical health. Administration of the Liver Frailty Index within routine clinical practice would allow clinicians to incorporate this objective metric of physical frailty into their decision-making and management plans for their patients, in the same way that they are already incorporating the other objective data that is available such as the vital signs, MELDNa score, and imaging studies (Figure 2). Our data demonstrate that the Liver Frailty Index is as vital a sign to a patient with cirrhosis as blood pressure or heart rate and can enhance the subjective assessments that clinicians must make about their patients, providing strong justification for the implementation of the Liver Frailty Index along with the traditional vital signs at every clinic visit.
Study Highlights.
WHAT IS CURRENT KNOWLEDGE
-
✓
Frailty is prevalent in patients with cirrhosis, particularly those awaiting liver transplantation, and is a critical determinant of mortality in this population, independent of liver disease severity.
-
✓
Frailty is most commonly incorporated into clinical decision-making through the clinician’s “eyeball test”, which is a subjective assessment of the patient’s global health status.
-
✓
The Liver Frailty Index was recently developed to standardize assessments of frailty using the combination of three objective, performance-based tests of physical function.
WHAT IS NEW HERE
-
✓
We demonstrate that the addition of the Liver Frailty Index to the subjective clinician assessment significantly improved mortality risk prediction, reclassifying 34% of patients.
-
✓
Our data strongly support the incorporation of the objective LFI to anchor our assessments of cirrhotic patients to enhance our decision-making.
Acknowledgments
We thank the hepatologists at UCSF for their willingness to provide their subjective global assessments of their patients’ health.
Financial support: This study was funded by an American College of Gastroenterology Junior Faculty Development Award, P30AG044281 (UCSF Older Americans Independence Center), and K23AG048337 (Paul B. Beeson Career Development Award in Aging Research). These funding agencies had no role in the analysis of the data or the preparation of this manuscript.
Footnotes
CONFLICT OF INTEREST
Potential competing interests: None.
Guarantor of the article: Jennifer C. Lai, MD, MBA.
Specific author contributions: Lai: Study concept and design, acquisition of data, analysis and interpretation of data, drafting of manuscript, critical revision of the manuscript for important intellectual content, statistical analysis, obtained funding, study supervision. Covinsky: Study concept and design, interpretation of data, critical revision of the manuscript for important intellectual content. McCulloch: Study design, analysis and interpretation of data, critical revision of the manuscript for important intellectual content. Feng: Study concept and design, interpretation of data, critical revision of the manuscript for important intellectual content.
References
- 1.Lai JC, Covinsky KE, Hayssen H, et al. Clinician assessments of health status predict mortality in patients with end-stage liver disease awaiting liver transplantation. Liver Int. 2015;35:2167–73. doi: 10.1111/liv.12792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:146–56. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 3.Lai JC, Dodge JL, Covinsky KE, et al. A novel Frailty Index for patients with end-stage liver disease. Hepatology. 2016;66:564–74. doi: 10.1002/hep.29219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lai JC, Feng S, Terrault NA, et al. Frailty predicts waitlist mortality in liver transplant candidates. Am J Transplant. 2014;14:1870–9. doi: 10.1111/ajt.12762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Weissenborn K, Ruckert N, Hecker H, et al. The number connection tests A and B: interindividual variability and use for the assessment of early hepatic encephalopathy. J Hepatol. 1998;28:646–53. doi: 10.1016/s0168-8278(98)80289-4. [DOI] [PubMed] [Google Scholar]
- 6.Guralnik JM, Simonsick EM, Ferrucci L, et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49:M85–94. doi: 10.1093/geronj/49.2.m85. [DOI] [PubMed] [Google Scholar]
- 7.Newman TB, Kohn MA. Evidence-Based Diagnosis. Cambridge University Press; USA: 2009. Reliability and measurement error; pp. 10–38. https://doi.org/10.1017/CBO9780511759512.003. [Google Scholar]
- 8.Vittinghoff E, Glidden DV, Shiboski SC, et al. Regression Methods in Biostatistics: Linear, Logistic, Survival, and Repeated Measures Models. 2. Springer; 2012. [Google Scholar]
- 9.Jepsen P, Vilstrup H, Andersen PK. The clinical course of cirrhosis: the importance of multistate models and competing risks analysis. Hepatology. 2015;62:292–302. doi: 10.1002/hep.27598. [DOI] [PubMed] [Google Scholar]
- 10.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496–509. [Google Scholar]
- 11.Pencina MJ, D'Agostino RB, Sr, Steyerberg EW. Extensions of net reclassification improvement calculations to measure usefulness of new biomarkers. Stat Med. 2011;30:11–21. doi: 10.1002/sim.4085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kruse JA, Thill-Baharozian MC, Carlson RW. Comparison of clinical assessment with APACHE II for predicting mortality risk in patients admitted to a medical intensive care unit. JAMA. 1988;260:1739–42. [PubMed] [Google Scholar]
- 13.Rocker G, Cook D, Sjokvist P, et al. Clinician predictions of intensive care unit mortality. Crit Care Med. 2004;32:1149–54. doi: 10.1097/01.ccm.0000126402.51524.52. [DOI] [PubMed] [Google Scholar]
- 14.Sinuff T, Adhikari NKJ, Cook DJ, et al. Mortality predictions in the intensive care unit: comparing physicians with scoring systems. Crit Care Med. 2006;34:878–85. doi: 10.1097/01.CCM.0000201881.58644.41. [DOI] [PubMed] [Google Scholar]
- 15.Copeland-Fields L, Griffin T, Jenkins T, et al. Comparison of outcome predictions made by physicians, by nurses, and by using the Mortality Prediction Model. Am J Crit Care. 2001;10:313–9. [PubMed] [Google Scholar]
- 16.McClish DK, Powell SH. How well can physicians estimate mortality in a medical intensive care unit? Med Decis Making. 1989;9:125–32. doi: 10.1177/0272989X8900900207. [DOI] [PubMed] [Google Scholar]
- 17.Marcin JP, Pollack MM, Patel KM, et al. Combining physician's subjective and physiology-based objective mortality risk predictions. Crit Care Med. 2000;28:2984–90. doi: 10.1097/00003246-200008000-00050. [DOI] [PubMed] [Google Scholar]
- 18.Lai JC, Dodge JL, Sen S, et al. Functional decline in patients with cirrhosis awaiting liver transplantation: results from the Functional Assessment in Liver Transplantation (FrAILT) Study. Hepatology. 2016;63:574–80. doi: 10.1002/hep.28316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Loza AJM, Junco JM, Prado CMM, et al. Muscle wasting is associated with mortality in patients with cirrhosis. Clin Gastroenterol Hepatol. 2012;10:166–73. 173.e1. doi: 10.1016/j.cgh.2011.08.028. [DOI] [PubMed] [Google Scholar]
- 20.Tandon P, Ney M, Irwin I, et al. Severe muscle depletion in patients on the liver transplant wait list: its prevalence and independent prognostic value. Liver Transpl. 2012;18:1209–16. doi: 10.1002/lt.23495. [DOI] [PubMed] [Google Scholar]
- 21.Vellas B, Fielding R, Bhasin S, et al. Sarcopenia trials in specific diseases: report by the International Conference on Frailty and Sarcopenia Research Task Force. J Frailty Aging. 2016;5:194–200. doi: 10.14283/jfa.2016.110. [DOI] [PubMed] [Google Scholar]
- 22.Lai JC, Covinsky KE, Dodge JL, et al. Development of a novel Frailty Index to predict mortality in patients with end-stage liver disease. Hepatology. 2017:1–35. doi: 10.1002/hep.29219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tapper EB, Finkelstein D, Mittleman MA, et al. Standard assessments of frailty are validated predictors of mortality in hospitalized patients with cirrhosis. Hepatology. 2015;62:584–90. doi: 10.1002/hep.27830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Samoylova ML, Covinsky KE, Haftek M, et al. Disability in patients with end-stage liver disease: results from the functional assessment in liver transplantation study. Liver Transpl. 2017;23:292–8. doi: 10.1002/lt.24684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guralnik JM, Ferrucci L, Simonsick EM, et al. Lower-extremity function in persons over the age of 70 years as a predictor of subsequent disability. N Engl J Med. 1995;332:556–61. doi: 10.1056/NEJM199503023320902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Prentis JM, Manas DMD, Trenell MI, et al. Submaximal cardiopulmonary exercise testing predicts 90-day survival after liver transplantation. Liver Transpl. 2012;18:152–9. doi: 10.1002/lt.22426. [DOI] [PubMed] [Google Scholar]
- 27.Carey EJ, Steidley DE, Aqel BA, et al. Six-minute walk distance predicts mortality in liver transplant candidates. Liver Transpl. 2010;16:1373–8. doi: 10.1002/lt.22167. [DOI] [PubMed] [Google Scholar]
- 28.McGee DL, Liao Y, Cao G, et al. Self-reported health status and mortality in a multiethnic US cohort. Am J Epidemiol. 1999;149:41–6. doi: 10.1093/oxfordjournals.aje.a009725. [DOI] [PubMed] [Google Scholar]
- 29.Tandon P, Tangri N, Thomas L, et al. A rapid bedside screen to predict unplanned hospitalization and death in outpatients with cirrhosis: a prospective evaluation of the Clinical Frailty Scale. Am J Gastroenterol. 2016;111:1759–67. doi: 10.1038/ajg.2016.303. [DOI] [PubMed] [Google Scholar]
- 30.Tandon P, Reddy KR, O'Leary JG, et al. A Karnofsky performance status-based score predicts death after hospital discharge in patients with cirrhosis. Hepatology. 2017;65:217–24. doi: 10.1002/hep.28900. [DOI] [PubMed] [Google Scholar]
- 31.Mathiowetz V, Weber K, Volland G, et al. Reliability and validity of grip and pinch strength evaluations. J Hand Surg Am. 1984;9:222–6. doi: 10.1016/s0363-5023(84)80146-x. [DOI] [PubMed] [Google Scholar]
- 32.Freire AN, Guerra RO, Alvarado B, et al. Validity and reliability of the short physical performance battery in two diverse older adult populations in Quebec and Brazil. J Aging Health. 2012;24:863–78. doi: 10.1177/0898264312438551. [DOI] [PubMed] [Google Scholar]