Abstract
Banked blood exhibits impairments in nitric oxide (NO)‐based oxygen delivery capability, reflected in rapid depletion of S‐nitrosohemoglobin (SNO‐Hb). We hypothesized that transfusion of even freshly‐stored blood used in pediatric heart surgery would reduce SNO‐Hb levels and worsen outcome. In a retrospective review (n = 29), the percent of estimated blood volume (% eBV) replaced by transfusion directly correlated with ventilator time and inversely correlated with kidney function; similar results were obtained in a prospective arm (n = 20). In addition, an inverse association was identified between SNO‐Hb and postoperative increase in Hb (∆Hb), reflecting the amount of blood retained by the patient. Both SNO‐Hb and ∆Hb correlated with the probability of kidney dysfunction and oxygenation‐related complications. Further, regression analysis identified SNO‐Hb as an inverse predictor of outcome. The findings suggest that SNO‐Hb and ∆Hb are prognostic biomarkers following pediatric cardiopulmonary bypass, and that maintenance of red blood cell‐derived NO bioactivity might confer therapeutic benefit.
Study Highlights
WHAT IS THE CURRENT KNOWLEDGE ON THE TOPIC?
✓ Pediatric patients who undergo cardiopulmonary bypass can receive large volumes of allogenic red blood cells. Transfusion of banked blood may enhance rather than correct deficits in tissue oxygenation, which may lead to organ dysfunction and worse postoperative outcome. This is because banked blood is depleted of S‐nitrosohemoglobin (SNO‐Hb), the main regulator of microvascular blood flow.
WHAT QUESTION DID THIS STUDY ADDRESS?
✓ Would transfusion of even freshly‐stored blood used in pediatric heart surgery reduce SNO‐Hb levels and worsen outcome?
WHAT THIS STUDY ADDS TO OUR KNOWLEDGE
✓ We linked declines in SNO‐Hb caused by intraoperative transfusion to reductions in tissue oxygenation, organ dysfunction, and worse outcomes in young cardiac surgery patients.
HOW THIS MIGHT CHANGE CLINICAL PHARMACOLOGY OR TRANSLATIONAL SCIENCE
✓ SNO‐Hb was prognostic for outcome, suggesting that it may be used as a biomarker of transfusion efficacy. S‐Nitrosylating agents that raise SNO‐Hb levels are currently undergoing human testing.
Congenital heart defects are the most frequent birth anomaly, with an occurrence rate close to 1% of all live deliveries.1 Within this group, at least one‐quarter of afflicted individuals will require surgical intervention early in life to correct the lesion. Neonatal and pediatric cardiopulmonary bypass (CPB) equipment and surgical techniques improved in concert with the adult technology during the mid‐part of the 20th century. As a result, the current prognosis for children with even the severest congenital defects is greatly improved, with 3–5 year survival rates of >70%.2
Advances in surgical methodology notwithstanding, CPB remains a significant stressor to the young patient and the need to administer banked blood is commonplace. Allogenic red blood cells (RBCs) are utilized to prime the bypass circuit, replace intraoperative blood loss, and maintain hemodynamic stability. As in other anemic settings, the administration of RBCs during CPB is premised on a direct correlation between the oxygen‐carrying capacity of blood and the delivery of oxygen to tissues, i.e., it is assumed that transfusion will improve tissue oxygenation. However, similar to adult cardiac populations, infants and neonates who receive RBCs have longer recovery periods and higher rates of adverse events than nontransfused cohorts.3, 4 A possible explanation is that the administration of stored blood may exacerbate rather than correct anemia‐induced deficits in tissue oxygenation.5
Tissue oxygen delivery is regulated by hypoxic vasodilation, a physiologic mechanism that couples local oxygen requirements to blood flow.6 RBCs serve as a principal transducer of this response by mediating the export of S‐nitrosothiol (SNO)‐based nitric oxide (NO) bioactivity. More specifically, NO is transported in RBCs by the conserved Cys residue at position 93 of the β chain (βCys93) in hemoglobin in the form of a SNO, i.e., S‐nitrosohemoglobin (SNO‐Hb).7 Low pO2 in tissues promotes the release of SNO‐based vasodilatory activity from RBCs to maintain tissue perfusion. The centrality of βCys93‐derived SNO in maintaining tissue oxygenation has recently been validated by strict genetic criteria,8 and is supported further by the demonstration of enhanced myocardial injury and mortality in the absence of βCys93 across different models of heart disease.9 This in turn has led to a reconceptualization of the respiratory cycle as a three‐gas system (O2/NO/CO2).10 Assessment of NO status provides a basis for understanding why increasing blood oxygen content (e.g., transfusion) can fail to improve tissue oxygenation;11 blood flow, not blood oxygen content, is the primary determinant of oxygen delivery under basal physiological conditions.12
A variety of conditions characterized by impairments in tissue oxygenation are associated with decreased bioavailability of RBC SNO,13, 14, 15 including transfusion.16, 17 Storage of blood leads to rapid losses in SNO‐Hb that are paralleled by declines in the ability of banked RBCs to effect hypoxic vasodilation.16 Administration of these SNO‐Hb‐depleted RBCs to anemic animals impairs microvascular blood flow, tissue oxygenation, and organ function (replicating the hypoxic pathophysiology exhibited by βCys93Ala, i.e., SNO‐deficient, mutant mice).8, 9 Administration of SNO‐Hb‐replete blood prevents these adverse events.17
Most practitioners strive to administer the “freshest” blood to their young patients. Yet the pediatric outcome data suggest that receipt of any allogenic RBCs,18 not expressly older blood,19 is associated with increased morbidity. Similarly, studies of fresh vs. aged blood in adults have not identified improvements in outcome.20, 21, 22 The loss of SNO‐Hb begins within hours of donation and is 80% depleted within 2 days.16, 23 Accordingly, administration of SNO‐depleted RBCs provides a plausible explanation for how transfusion may worsen outcome. With this in mind, we aimed to first determine if RBC transfusion was associated with adverse events in our population of neonatal congenital heart patients. As the answer was yes, we followed the retrospective review with a prospective study to determine the effects of transfusion on SNO‐Hb levels. We predicted that transfusion‐induced reductions in SNO‐Hb would portend worse outcome, including impaired organ function and increased incidence of oxygenation‐related complications.
METHODS
Study overview
This investigation was a single‐site observational study. The research protocol was approved by the Institutional Review Board (IRB) of University Hospitals Cleveland Medical Center and written informed consent for study participation in the prospective arm was obtained from the parent(s) or legal guardian(s) of each patient prior to surgery (the IRB waived the need to obtain consent for the retrospective chart review). The target population was neonates and young children (<12 months of age) anticipated to receive packed RBCs during open‐heart surgery with CPB for repair of congenital heart disease. Older children and patients undergoing redo procedures were excluded.
The chart review retrieved demographic and perioperative data (RBC transfusion volumes, clinical chemistries, ventilator times, complications, length of stay, etc.) for children operated on between November 2008 and December 2009. The prospective arm enrolled subjects between January 2011 and April 2013.
Procedures
An established bypass procedure (including muscle relaxation with vecuronium and antifibrinolytic therapy with aminocaproic acid) was used for all patients with the surgical techniques dictated by the cardiac anomaly. All CPB surgeries in the prospective study were conducted by the same surgeon (P.C.K.) and perfusion team (J.O. and R.S.) (see Supplemental Content for details). A dual‐lead INVOS near infrared spectroscopy (NIRS) system (Medtronic, Minneapolis, MN) was used to monitor cerebral and kidney oxygenation.24, 25 To determine SNO‐Hb levels, blood samples (∼2 mL) were drawn from the arterial port of the oxygenator during set points of the surgery and from an indwelling arterial line on postoperative Day 1. (Blood sampling and RBC administration did not occur contemporaneously.) No samples were obtained from percutaneous needle sticks, so we do not have preinduction SNO‐Hb or blood gas values. The decision to remove the arterial line was made independently of SNO sampling needs (per hospital protocol, this typically occurred on postoperative Day 1), which precluded procurement of additional arterial blood samples for this minimally invasive study. Postoperative patient management was directed by the pediatric ICU staff who were unaware of the study goals and all other blood samplings were done at the direction of the clinical team. RBC SNO‐Hb levels were quantified offline and the resultant values were not used to direct any child's clinical care. Additional patient details and information on postoperative course were collected from the medical record.
RBC SNO‐Hb measurement protocol
Photolysis‐chemiluminescence was used to quantify SNO‐Hb levels.16, 23, 26, 27 The method has been well validated for selective determination of RBC HbNO without artificial contamination from other nitrosative species, including nitrite and nitrate.28, 29 Within 1 h of procurement, the arterial blood samples were spun at low speed, then, after decanting, the pelleted RBCs were washed twice with 0.1 mM diethylene‐triaminepentaacetic acid (DTPA) in phosphate‐buffered saline (PBS) at pH 7.4 and then lysed in excess deionized water (1:4 volume ratio x 10 min) containing 0.1 mM DTPA. Lysates were centrifuged (20,000g for 10 min) to separate membranes and cytosol. Membranes were dissolved in Triton X‐100 (2%) in PBS, and Hb was desalted by centrifugation (3,000g for 1 min) through Sephadex G‐25 spin columns (Pharmacia, Uppsala, Sweden). The Hb samples were then stored at –80°C for batch analysis. The amount of Hb in the eluents was determined spectrophotometrically, adjusted to a final concentration of 400 μM, and then incubated with either PBS alone or with sixfold molar excess of mercuric chloride (which selectively cleaves thiol‐bound NO groups). Standard curves were generated daily with S‐nitrosoglutathione. Concentrations of SNO‐Hb and Hb[FeNO] were calculated based on the difference between the amount of NO liberated by UV light in the absence (SNO‐Hb plus Hb[FeNO]) vs. presence of mercuric chloride (Hb[FeNO]).13, 30
Statistics
Interval and ratio data are expressed as mean ± standard deviation (SD) and ordinal data are expressed as median ± interquartile range. To assess kidney function, estimated glomerular filtration rate (eGFR) was calculated from serum chemistries using the formula eGFR = 0.45 x [height/Scr] with height in centimeters and serum creatinine (Scr) in mg/dl.31 Estimated blood volume (eBV) for each patient was determined by body weight and age.32 Where appropriate, log‐transformation was used to satisfy conditions of data normality. Standard parametric methods were used to check for differences over time (e.g., repeated‐measures anaylsis of variance (ANOVA) with Dunnett's post‐hoc testing), while linear regression was used to test for associations. A logistic stepwise regression model was employed to construct a predictive model for major morbidity (described in detail in the Supplement).
RESULTS
Demographic data and general patient outcomes
Data from 29 clinical records were collated in the retrospective study while 20 pediatric heart patients were enrolled in the prospective arm; their demographic and operative data are presented in Supplemental Table S1. The two patient populations and the procedural aspects of the surgeries were similar. Importantly, both groups demonstrated negative responses to the intraoperative receipt of RBCs. The amount of blood received (% of eBV replaced) directly correlated with increased postoperative ventilator time (Supplemental Figure S1A and S1B; r = 0.676, P = 0.0008 for the retrospective group and r = 0.595, P = 0.005 for the prospective cohort) and increased intensive care unit (ICU) stay in the prospective cohort (Figure S1C; r = 0.542, P = 0.012). (Note: information on ICU duration was not recorded in the files of the retrospective group.) Organ function was also impacted negatively by transfusion in the retrospective group, where receipt of more blood was associated with worse kidney function, i.e., declines in eGFR (Figure S1D; r = –0.392, P = 0.04). This functional consequence was explored further in the prospective study.
Intraoperative transfusion and tissue oxygenation
We predicted that receipt of SNO‐Hb‐depleted RBCs would adversely impact tissue oxygenation and support for this hypothesis was demonstrated in the prospective arm. First, the % of eBV replaced was inversely correlated with kidney tissue oxygenation (StO2) at the end of surgery (Figure 1 a; r = –0.722, P = 0.0003); a similar albeit weaker inverse association with brain StO2 was also observed (r = –0.317, P = 0.172). Second, transfusion increased RBC mass but no correlation was found between kidney StO2 and arterial oxygen content (Figure 1 b; r = –0.122, P = 0.651). Third, as with the retrospective analysis, the amount of blood transfused (% of eBV replaced) was inversely correlated with eGFR (Figure 1 c; r = –0.533, P = 0.015), reflecting the importance of microvascular flow on tissue oxygenation. Taken together, these data demonstrate the negative effect of transfusion on postoperative renal function as a consequence of decreased intraoperative kidney StO2.
Figure 1.
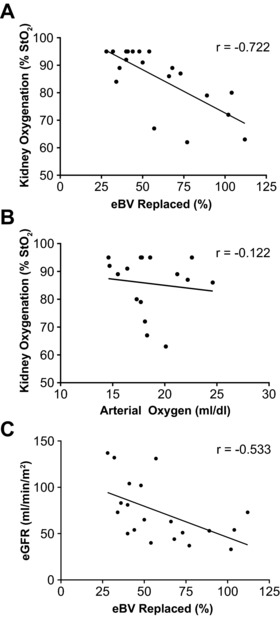
Transfusion, tissue oxygenation, and organ function. (a) The % eBV replaced by intraoperative transfusion was inversely correlated with kidney StO2 at the end of surgery (n = 20; r = –0.722, P = 0.0003). (b) There was no correlation between kidney StO2 and arterial blood oxygen content (r = –0.122, P = 0.651). (c) Scatterplot depicts the inverse correlation between eGFR and % eBV replaced (r = –0.533, P = 0.015).
SNO‐Hb, transfusion, and outcome
The negative association between intraoperative transfusion and StO2 suggested a role for SNO‐Hb, which we tracked prospectively. Initiating bypass alone resulted in a rise in arterial SNO‐Hb levels (Figure 2 a). Specifically, SNO‐Hb increased by more than 20% (from 1.45 ± 0.95 to 1.74 ± 1.09 moles SNO‐Hb per moles Hb x 10−3) after patients were put on pump, then continued to rise over the ensuing 24 h, peaking at 2.43 ± 1.08 per Hb x 10−3 (before removal of arterial access). However, this trend showed significant interpatient variability. To assess whether the variation in SNO‐Hb was related to transfusion volume we first sought a correlation between SNO‐Hb and % eBV replaced, but none was found (Figure 2 b; r = –0.006, P = 0.979). Rather, postop SNO‐Hb concentrations were in fact strongly correlated with amounts of transfused RBCs retained by the subject as measured by the increase between pre‐ and posttransfusion Hb (∆Hb) (Figure 2 c; r = –0.573, P = 0.010). Thus, individuals whose blood was diluted the least by allogenic RBCs had the highest levels of postoperative SNO‐Hb and vice versa. In addition, there was no correlation between the RACHS score (Risk Adjustment for Congenital Heart Surgery)33 and transfusion volume, implying that the impact of RBC administration on outcome was independent of the neonates’ preoperative condition or surgical complexity.
Figure 2.
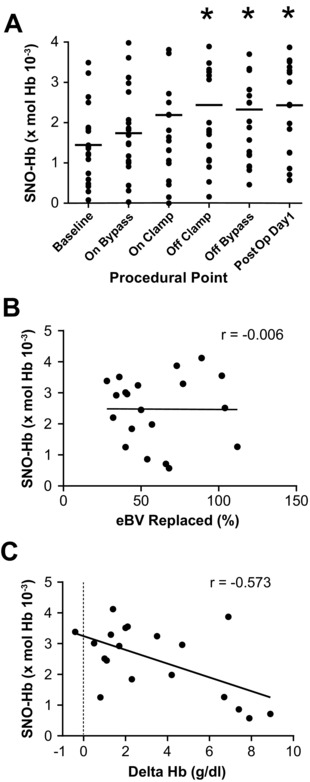
SNO‐Hb, CPB, and transfusion. (a) Circulating RBC SNO‐Hb concentrations in pediatric patients (n = 20; group means are designated by the bars) at various procedural points before, during, and after CPB. SNO‐Hb levels increased after going on bypass and continued to rise into the postoperative period. *Significant difference compared with baseline, P < 0.05, as determined by repeated‐measures ANOVA followed by Dunnet's test. (b) There was no relationship between SNO‐Hb levels and % eBV replaced. (c) The increase in SNO‐Hb correlated inversely with the magnitude of the pre‐to‐posttransfusion increase in Hb (∆Hb) (r = –0.573, P = 0.010). Retention of more blood (i.e., greater increase in postop Hb) was thus associated with lower SNO‐Hb.
Next we sought a relationship between SNO‐Hb and patient outcomes. Notably, a positive linear relationship was found between eGFR and SNO‐Hb levels (r = 0.464, P = 0.039), consistent with the idea that SNO‐Hb maintains kidney oxygenation and with the finding that transfusion, which lowered SNO‐Hb concentration, was associated with a decline in kidney StO2 (Figure 1 a). We compiled a list of postoperative adverse events that may be linked directly to disruptions in oxygen delivery (Figure 3 a) and found a strong positive correlation with ∆Hb (Figure 3 c; r = 0.587, P = 0.008) and a strong inverse correlation with SNO‐Hb (Figure 3 D; r = –0.695, P = 0.0007). In contrast, we found only weak correlations between complication risk and % of eBV replaced (Figure 3 b; r = 0.392, P = 0.08). Furthermore, a logistic regression model (defined in Supplemental Tables S2 and S3) identified an inverse correlation between SNO‐Hb levels and postoperative complications, including all‐cause morbidity (Table 1). Thus, postoperative SNO‐Hb was a strong prognostic biomarker following intraoperative transfusion.
Figure 3.
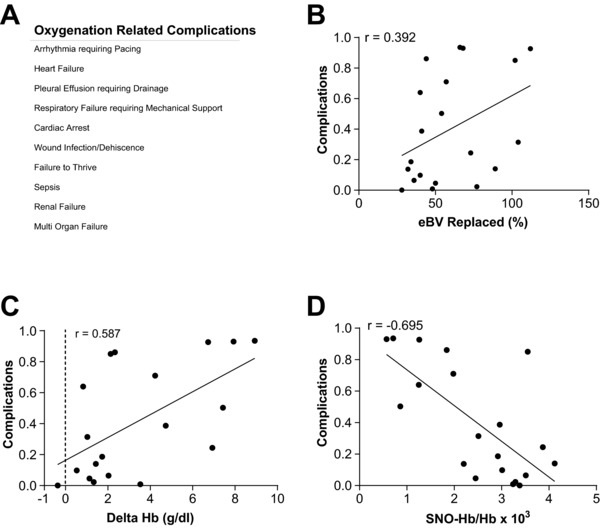
Oxygenation‐related complications. (a) Table enumerating the oxygenation‐related complications. (b) A scatterplot depicting the weak correlation between % eBV replaced and probability of complications (n = 20, r = 0.392, P = 0.08). (c) ∆Hb demonstrates a positive correlation with probability of complications (n = 20, r = 0.587, P = 0.008). (d) SNO‐Hb has a negative correlation with probability of complications (n = 20, r = –0.695, P = 0.0007).
Table 1.
Logistical regression analysis between subject factors
| Major morbiditya | β | SE | Wald (χ2) | df | P | eβ (odds ratio) |
| Postop SNO‐Hbb | –1.437 | 0.693 | 4.293 | 1 | 0.03 | 0.238 |
| Constant | 12.297 | 5.658 | 4.723 | 1 | 0.30 | |
| Goodness of fit test | R2 | χ2 | df | P | ||
| Hosmer and Lemeshow test | 8.088 | 8 | 0.425 | |||
| Cox and Snell | 0.445 | |||||
| Nagelkerke | 0.602 |
Major morbidity was coded as yes or no.
Postoperative S‐nitrosohemoglobin.
DISCUSSION
Donated RBCs undergo progressive time‐dependent changes in RBC integrity and function, including loss of SNO‐Hb, which impairs the ability of RBCs to oxygenate tissues.8, 17 The loss of SNO‐Hb begins within hours of donation,16, 23 which is consistent with reports that even freshly‐processed blood may negatively impact tissue oxygenation34 and patient outcomes.20, 21, 22 Deficiency in SNO‐Hb is causally linked to renal injury,17, 35 a major adverse consequence of intraoperative blood transfusion.36 The current findings add to this perspective by suggesting a mechanistic basis for transfusion‐related tissue injury: lower SNO‐Hb levels reduce kidney StO2 and portend adverse patient outcomes, including reductions in eGFR.
We had anticipated that neonatal bypass would result in a net decline in SNO‐Hb because SNO‐depleted RBCs were used in the priming solution; however, the opposite was in fact observed. As a group, the subjects exhibited an early increase in SNO‐Hb concentration. We attribute this rise to initial improvements in blood oxygenation as a result of going on pump (especially in the cyanotic babies); oxygenated Hb favors the production of SNO‐Hb.7, 12 At the same time, changes in SNO‐Hb concentrations were related to the amounts of allogenic blood received: receipt and retention of higher volumes of stored RBCs was associated with lower SNO‐Hb.
The administration and retention of RBCs deficient in SNO‐Hb (% of eBV replaced and ∆Hb, respectively) provide a mechanistic explanation for posttransfusion complications.5 This can be understood by appreciating that erythrocytes pass through the microcirculation in line.37 Banked RBCs, unable to elicit vasodilation, will get stuck and thus impede flow to adversely influence oxygen delivery—an interpretation supported also by prior findings that administration of even small volumes of SNO‐Hb‐depleted RBCs can decrease organ blood flow and tissue pO2.16, 17 This concept of microvascular “plugging” is also consistent with empiric evidence that SNO‐deficient RBCs can adhere to endothelial linings to impair oxygenation.38
Subjects with lower SNO‐Hb levels had higher postoperative Hb (i.e., administration of SNO‐depleted Hb diluted the SNO‐replete Hb), and both low SNO‐Hb and high Hb predicted worse outcomes. While it is unclear if individuals might have tolerated a more conservative transfusion strategy (transfusion triggers in pediatric surgical patients are ill‐defined) there is accumulating evidence that restricting transfusion might be beneficial in some patients. In a trial of noncyanotic pediatric bypass subjects, dropping the transfusion threshold to 8.0 g/dl (from 10.8) reduced total hospital length of stay.18 Conceivably, restrictive strategies act to preserve SNO‐Hb levels. To this end, clinical13 and preclinical trials35, 39, 40 have demonstrated therapeutic benefits of Hb renitrosylation, which, in the setting of transfusion, act to enhance oxygen delivery.17 As such, the current findings support a follow‐on clinical trial to determine if perioperative renitrosylation therapy during neonatal heart surgery could improve outcomes.
We recognize that the correlative analysis of the prospectively collected data is a potential weakness of this study, and the multivariable analysis is weakened by the event sample size. However, the matching findings from both the retrospective chart review (and other bypass studies) and the preclinical studies8, 9 somewhat render moot these concerns. In addition, we note that the amount of blood transfused matched well with other pediatric trials,18 StO2 was tracked in real time (NIRS has been used frequently to noninvasively monitor kidney StO2 and predict renal injury in young cardiac patients),24, 25 and our transfusion‐related morbidities matched literature reports in this patient population.3, 4, 41 Moreover, the identification of SNO‐Hb as the determinant of outcome is consistent with a growing body of literature connecting deficits in RBC SNO‐Hb to pathologies of oxygenation.42
In summary, we have linked dysregulated SNO homeostasis caused by large‐volume intraoperative transfusion to reductions in tissue oxygenation and adverse events. We have also shown that SNO‐Hb levels are inversely correlated with kidney function and all‐cause morbidity, suggesting its utility as both a prognostic biomarker and target for therapeutic intervention. Together, our findings provide clinical support for the postulate that defects in the oxygen‐delivery function of stored RBCs, reflected in impairments in SNO‐Hb based vasoregulation, contribute to the deleterious effects of allogenic blood transfusion.
Conflict of Interest/Disclosure
Dr. Stamler has a financial interest in Nivalis Therapeutics, Adamas Pharma, and Vindica Pharm. Dr. Reynolds has a financial interest in Miach Medical Innovations. Drs. Stamler and Reynolds hold patents related to renitrosylation of blood, some of which have been licensed for commercial development. The institution is aware of these conflicts and appropriate management plans are in place. The other authors have no conflicts of interest relevant to this article to disclose.
Supporting information
1. Supplemental Content – Detailed Methods
2. Supplemental Content – Additional Results
Table S1. Cohort Demographic Information
Table S2. Types of Surgical Procedures
Table S3: Logistical Regression Analysis between Subject Factors
3. Figure S1. Retrospective Analysis of Blood Volume Replaced and Complications
A) The percent of estimated blood volume (eBV) replaced by intraoperative transfusion in the retrospective cohort was found to increase the time to extubation (n = 29; r = 0.676, P = 0.0008). B) Similar findings are depicted for the prospective cohort where time spent intubated also directly correlated with eBV (%) (n = 20, r = 0.595, P = 0.005). C) Scatter plot which depicts the correlation between eBV (%) and time spent in the ICU for the prospective cohort (n = 20, r = –0.542, P = 0.012), indicating higher transfusion volumes equating with worse outcomes. D) Receipt of more blood (measured by eBV) in the retrospective cohort was found to impact organ function as depicted by the negative correlation with eGFR (n = 29, P = –0.392, P = 0.04).
Acknowledgments
We thank the patients (and their parents/guardians) of the Division of Pediatric Cardiothoracic Surgery at Rainbow Babies & Children's Hospital who volunteered to participate in this study. We also thank Deborah West, MSN, for her expert assistance with compiling the clinical data.
Funding
This work was sponsored in part by NIH grants HL091876, HL095463 and HL126900, Defense Advanced Research Projects Agency Contracts N66001‐10‐C‐2015 and N66001‐13‐C‐4054, and grants from the Rainbow Babies & Children's Hospital Research Fund and from the Clinical and Translational Science Collaborative of Cleveland, 4UL1TR000439 funded by the National Center for Advancing Translational Sciences component of the National Institutes of Health and NIH roadmap for Medical Research. The publication's contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
Author Contributions
F.M., J.D.R., and J.S.S. wrote the article; D.T.H., J.D.R., and J.S.S. designed the research; A.J.C, P.C.K., F.M., J.O., and R.S. performed the research; F.M. and J.D.R. analyzed the data.
Current address for F. Matto: University of Iowa, Iowa City, IA. Current address for P.C. Kouretas: UCSF School of Medicine, San Francisco, CA.
References
- 1. van der Linde, D. et al Birth prevalence of congenital heart disease worldwide: A systematic review and meta‐analysis. J. Am. Coll. Cardiol. 58, 2241–2247 (2011). [DOI] [PubMed] [Google Scholar]
- 2. Feinstein, J.A. et al Hypoplastic left heart syndrome: Current considerations and expectations. J. Am. Coll. Cardiol. 59, S1‐42 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Salvin, J.W. et al Blood transfusion after pediatric cardiac surgery is associated with prolonged hospital stay. Ann. Thorac. Surg. 91, 204–210 (2011). [DOI] [PubMed] [Google Scholar]
- 4. Kneyber, M.C. et al Transfusion of leukocyte‐depleted RBCs is independently associated with increased morbidity after pediatric cardiac surgery. Pediatr. Crit. Care Med. 14, 298–305 (2013). [DOI] [PubMed] [Google Scholar]
- 5. Reynolds, J.D. , Hess, D.T. & Stamler, J.S. The transfusion problem: Role of aberrant S‐nitrosylation. Transfusion 51, 852–858 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ross, J.M. , Fairchild, H.M. , Weldy, J. & Guyton, A.C. Autoregulation of blood flow by oxygen lack. Am. J. Physiol. 202, 21–24 (1962). [DOI] [PubMed] [Google Scholar]
- 7. Allen, B.W. , Stamler, J.S. & Piantadosi, C.A. Hemoglobin, nitric oxide and molecular mechanisms of hypoxic vasodilation. Trends Mol. Med. 15, 452–460 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zhang, R. et al Hemoglobin βcys93 is essential for cardiovascular function and integrated response to hypoxia. Proc. Natl. Acad. Sci. U. S. A. (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zhang, R. , Hess, D.T. , Reynolds, J.D. & Stamler, J.S. Hemoglobin S‐nitrosylation plays an essential role in cardioprotection. J. Clin. Invest. 126, 4654–4658 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Doctor, A. & Stamler, J.S. Nitric oxide transport in blood: A third gas in the respiratory cycle. Compr. Physiol. 1, 541–568 (2011). [DOI] [PubMed] [Google Scholar]
- 11. Hodges, A.N. , Delaney, S. , Lecomte, J.M. , Lacroix, V.J. & Montgomery, D.L. Effect of hyperbaric oxygen on oxygen uptake and measurements in the blood and tissues in a normobaric environment. Br. J. Sports Med. 37, 516–520 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Singel, D.J. & Stamler, J.S. Chemical physiology of blood flow regulation by red blood cells: The role of nitric oxide and S‐nitrosohemoglobin. Annu. Rev. Physiol. 67, 99–145 (2005). [DOI] [PubMed] [Google Scholar]
- 13. McMahon, T.J. et al A nitric oxide processing defect of red blood cells created by hypoxia: Deficiency of S‐nitrosohemoglobin in pulmonary hypertension. Proc. Natl. Acad. Sci. U. S. A. 102, 14801–14806 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Pawloski, J.R. , Hess, D.T. & Stamler, J.S. Impaired vasodilation by red blood cells in sickle cell disease. Proc. Natl. Acad. Sci. U. S. A. 102, 2531–2536 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. James, P.E. , Lang, D. , Tufnell‐Barret, T. , Milsom, A.B. & Frenneaux, M.P. Vasorelaxation by red blood cells and impairment in diabetes: Reduced nitric oxide and oxygen delivery by glycated hemoglobin. Circ. Res. 94, 976–983 (2004). [DOI] [PubMed] [Google Scholar]
- 16. Reynolds, J.D. et al S‐Nitrosohemoglobin deficiency: A mechanism for loss of physiological activity in banked blood. Proc. Natl. Acad. Sci. U. S. A. 104, 17058–17062 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Reynolds, J.D. et al S‐Nitrosylation therapy to improve oxygen delivery of banked blood. Proc. Natl. Acad. Sci. U. S. A. 110, 11529–11534 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. de Gast‐Bakker, D.H. et al Safety and effects of two red blood cell transfusion strategies in pediatric cardiac surgery patients: A randomized controlled trial. Intens. Care Med. 39, 2011–2019 (2013). [DOI] [PubMed] [Google Scholar]
- 19. Mou, S.S. et al Fresh whole blood versus reconstituted blood for pump priming in heart surgery in infants. N. Engl. J. Med. 351, 1635–1644 (2004). [DOI] [PubMed] [Google Scholar]
- 20. Steiner, M.E. et al Effects of red‐cell storage duration on patients undergoing cardiac surgery. N. Engl. J. Med. 372, 1419–1429 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lacroix, J. et al Age of transfused blood in critically ill adults. N. Engl. J. Med. 372, 1410–1418 (2015). [DOI] [PubMed] [Google Scholar]
- 22. Heddle, N.M. et al Effect of short‐term vs. long‐term blood storage on mortality after transfusion. N. Engl. J. Med. 375, 1937–1945 (2016). [DOI] [PubMed] [Google Scholar]
- 23. Bennett‐Guerrero, E. et al Evolution of adverse changes in stored RBCs. Proc. Natl. Acad. Sci. U. S. A. 104, 17063–17068 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Owens, G.E. , King, K. , Gurney, J.G. & Charpie, J.R. Low renal oximetry correlates with acute kidney injury after infant cardiac surgery. Pediatr. Cardiol. 32, 183–188 (2011). [DOI] [PubMed] [Google Scholar]
- 25. Ortmann, L.A. et al Use of near‐infrared spectroscopy for estimation of renal oxygenation in children with heart disease. Pediatr. Cardiol. 32, 748–753 (2011). [DOI] [PubMed] [Google Scholar]
- 26. McMahon, T.J. & Stamler, J.S. Concerted nitric oxide/oxygen delivery by hemoglobin. Methods Enzymol. 301, 99–114 (1999). [DOI] [PubMed] [Google Scholar]
- 27. McMahon, T.J. et al Nitric oxide in the human respiratory cycle. Nat. Med. 8, 711–717 (2002). [DOI] [PubMed] [Google Scholar]
- 28. Stamler, J.S. S‐Nitrosothiols in the blood: roles, amounts, and methods of analysis. Circ. Res. 94, 414–417 (2004). [DOI] [PubMed] [Google Scholar]
- 29. Hausladen, A. et al Assessment of nitric oxide signals by triiodide chemiluminescence. Proc. Natl. Acad. Sci. U. S. A. 104, 2157–2162 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Pawloski, J.R. , Hess, D.T. , Stamler, J.S. Export by red blood cells of nitric oxide bioactivity. Nature 409, 622–626 (2001). [DOI] [PubMed] [Google Scholar]
- 31. Schwartz, G.J. & Work, D.F. Measurement and estimation of GFR in children and adolescents. Clin. J. Am. Soc. Nephrol. 4, 1832–1843 (2009). [DOI] [PubMed] [Google Scholar]
- 32. Limited, C.‐G. , Diem, K. & Lentner, C. Scientific Tables. 7th ed ed. (Geigy Pharmaceuticals; Macclesfield, UK; 1970) [Google Scholar]
- 33. Jenkins, K.J. et al Consensus‐based method for risk adjustment for surgery for congenital heart disease. J. Thorac. Cardiovasc. Surg. 123, 110–118 (2002). [DOI] [PubMed] [Google Scholar]
- 34. Tsai, A.G. , Cabrales, P. & Intaglietta, M. Microvascular perfusion upon exchange transfusion with stored red blood cells in normovolemic anemic conditions. Transfusion 44, 1626–1634 (2004). [DOI] [PubMed] [Google Scholar]
- 35. Shimazutsu, K. et al Inclusion of a nitric oxide congener in the insufflation gas repletes S‐nitrosohemoglobin and stabilizes physiologic status during prolonged carbon dioxide pneumoperitoneum. Clin. Transl. Sci. 2, 405–412 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Karkouti, K. et al Influence of erythrocyte transfusion on the risk of acute kidney injury after cardiac surgery differs in anemic and nonanemic patients. Anesthesiology 115, 523–530 (2011). [DOI] [PubMed] [Google Scholar]
- 37. Japee, S.A. , Pittman, R.N. & Ellis, C.G. Automated method for tracking individual red blood cells within capillaries to compute velocity and oxygen saturation. Microcirculation 12, 507–515 (2005). [DOI] [PubMed] [Google Scholar]
- 38. Riccio, D.A. et al Renitrosylation of banked human red blood cells improves deformability and reduces adhesivity. Transfusion 55, 2452–2463 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Auten, R.L. et al Inhaled ethyl nitrite prevents hyperoxia‐impaired postnatal alveolar development in newborn rats. Am. J. Respir. Crit. Care Med. 176, 291–299 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Sheng, H. et al Pharmacologically augmented S‐nitrosylated hemoglobin improves recovery from murine subarachnoid hemorrhage. Stroke 42, 471–476 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kipps, A.K. , Wypij, D. , Thiagarajan, R.R. , Bacha, E.A. & Newburger, J.W. Blood transfusion is associated with prolonged duration of mechanical ventilation in infants undergoing reparative cardiac surgery. Pediatr. Crit. Care Med. 12, 52–56 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Haldar, S.M. & Stamler, J.S. S‐Nitrosylation: Integrator of cardiovascular performance and oxygen delivery. J. Clin. Invest. 123, 101–110 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
1. Supplemental Content – Detailed Methods
2. Supplemental Content – Additional Results
Table S1. Cohort Demographic Information
Table S2. Types of Surgical Procedures
Table S3: Logistical Regression Analysis between Subject Factors
3. Figure S1. Retrospective Analysis of Blood Volume Replaced and Complications
A) The percent of estimated blood volume (eBV) replaced by intraoperative transfusion in the retrospective cohort was found to increase the time to extubation (n = 29; r = 0.676, P = 0.0008). B) Similar findings are depicted for the prospective cohort where time spent intubated also directly correlated with eBV (%) (n = 20, r = 0.595, P = 0.005). C) Scatter plot which depicts the correlation between eBV (%) and time spent in the ICU for the prospective cohort (n = 20, r = –0.542, P = 0.012), indicating higher transfusion volumes equating with worse outcomes. D) Receipt of more blood (measured by eBV) in the retrospective cohort was found to impact organ function as depicted by the negative correlation with eGFR (n = 29, P = –0.392, P = 0.04).


