Figure 1.
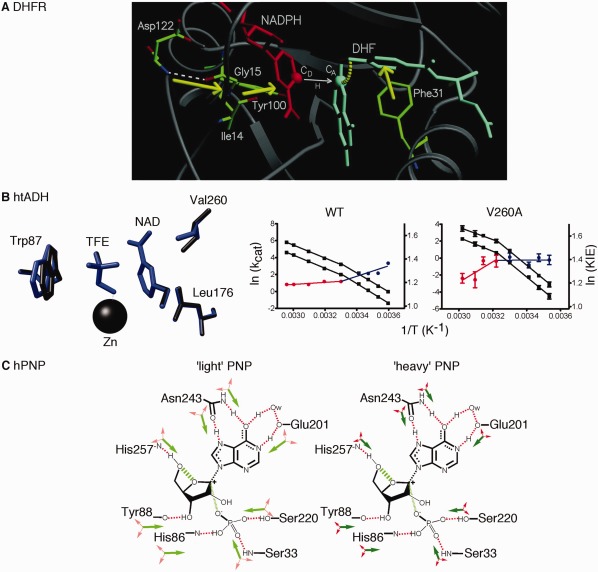
Promoting motions proposed to be important in the compression of the reaction coordinate in enzyme catalyzed reactions. A. Molecular dynamics simulations suggest a network of motions in dihydrofolate reductase that sterically compresses the reaction coordinate and gates the distance between hydride donor (CD) and acceptor (CA) atoms. Reprinted with permission from Ref. 19. B. Formation of small pockets near the NAD cofactor of thermophilic alcohol dehydrogenase through the substitution of Val260 (or Leu176) with Ala leads to changes in the catalytic turnover rate (kcat) and the H/D kinetic isotope effect (KIE) for hydride transfer. The kcat values at different temperatures for hydrogen and deuterium transfer are shown in black (plotted as a function of the left axis), and red and blue indicate the KIE (=kcat,H/kcat,D) highlighting the temperature break in Arrhenius behavior around 30°C (plotted as a function of the right axis). The WT and V260A enzymes have opposing behaviors in the temperature dependence of the KIE. In the V260A variant, hydride tunneling is less important at low temperatures (represented by the blue line) because preorganization is disrupted. At high temperatures (represented by the red line), hydrogen tunneling is more important, but the created cavity increases the need for donor‐acceptor distance sampling (i.e., reorganization is also disrupted). Adapted with permission from Ref. 48. C. Proposed mass‐altered bond vibrational modes in the 1H, 12C, 14N “light” and 2H, 13C, 15N “heavy” human purine nucleoside phosphorylase enzymes. Green arrows tend to promote transition‐state barrier crossing, and red arrows are orthogonal to the reaction coordinate. In the “heavy” enzyme, local motions are reduced, as indicated by the smaller arrows, including those proposed to be important for compressing the distance between the O5’ and O4’ oxygens of the ribose ring, as indicated by the hashed green line. One key motion involves His257; amino acid changes designed to less sterically constrain His257 lead to mass‐independent barrier crossing rates. Adapted with permission from Ref. 56.
