Figure 5.
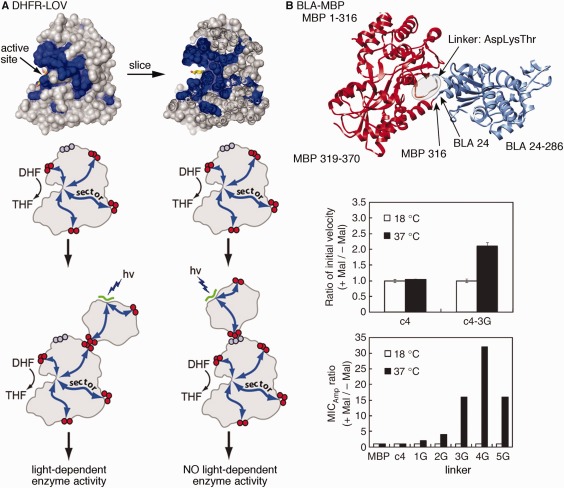
Chimeric enzymes to control structural dynamics and catalytic function according to external stimuli. A. Statistical coupling analysis was used to identify surface‐exposed network residues in E. coli dihydrofolate reductase and the light‐responsive LOV domain. Engineering of chimeric enzymes to join these positions results in light‐dependent catalytic conversion of dihydrofolate (DHF) to tetrahydrofolate (THF); use of other non‐network, surface‐exposed sites does not lead to light‐dependent enzyme activity. Reprinted from Ref. 97 with permission from Cell Press. B. Engineering of chimeric β‐lactamase‐maltose binding proteins. The c4 fusion protein as shown in the ribbon diagram is not responsive to the presence of maltose, but the introduction of additional glycine residues into the linker, which presumably increases molecular flexibility, led to maltose‐ and temperature‐dependent catalytic activity and biological response (according to ampicillin minimum inhibitory concentration, MIC). Reprinted with permission from Ref. 163.
