Abstract
Systemic lupus erythematosus (SLE) is a multisystem disorder, which occurs mostly in young women. However, late‐onset SLE does exist and sometimes presents with an atypical, diversified course. We describe an 85‐year‐old woman who was admitted to our hospital for lower extremity edema and hand grip weakness. Chest computed tomography scan 4 days after admission demonstrated rapid accumulation of pleural and pericardial effusions, which did not exist on admission. She was diagnosed with pleuritis and pericarditis associated with very‐late‐onset SLE. Methylprednisolone pulse therapy resulted in a drastic improvement in serositis. Our case exemplifies the fact that patients with late‐onset SLE sometimes follow an atypical course, which makes the clinical diagnosis difficult.
Keywords: pericarditis, pleuritis, serositis, very‐late‐onset systemic lupus erythematosus
1. INTRODUCTION
Systemic lupus erythematosus (SLE) is a multisystem inflammatory disorder with a broad spectrum of clinical and laboratory manifestations, which typically occurs among juvenile and adult women. Although autoimmunity plays a major role in the onset of SLE, details of the pathogenesis of SLE are still under intense investigation.1 The most common age at diagnosis is in the 20s and the 30s. SLE can present after age 50, but only rarely. It is indisputable that very‐late‐onset SLE after age 80 is even rarer.2, 3 We herein report a case of an 85‐year‐old woman with severe serositis associated with very‐late‐onset SLE, who was successfully treated with steroid therapy.
2. CASE REPORT
An 85‐year‐old woman was referred to our hospital because of a 2‐month history of bilateral lower extremity edema and hand grip weakness. Her past medical history was significant for intravascular large B‐cell lymphoma (IVLBCL), which was diagnosed by bone marrow biopsy 10 years ago at another hospital and achieved complete remission after chemotherapy.
On admission, her body temperature was 37.6°C and other vital signs were unremarkable. Physical examination revealed conjunctival pallor, a systolic murmur along the right sternal border II, mild bilateral lower extremity pitting edema, and pain and swelling in the proximal interphalangeal (PIP) joints. There was no jugular venous distention, palpable lymphadenopathy, Raynaud's phenomenon, or skin eruptions. Laboratory investigation revealed microcytic anemia and elevation of the erythrocyte sedimentation rate (ESR), levels of D‐dimer, fibrin degradation product (FDP), lactate dehydrogenase (LDH), and C‐reactive protein (CRP), while renal and thyroid function tests were normal (Table 1). Urinalysis was normal as well. Although echocardiography showed moderate aortic valve stenosis, left ventricular systolic/diastolic functions were not impaired. Plain and contrast‐enhanced computed tomography (CT) showed no pleural effusion (Figure 1, day 1) or deep venous thromboembolism (DVT).
Table 1.
Laboratory data obtained at our hospital on admission
| Hematology | Biochemistry | Ca | 9.4 mg/dL | ||
| WBC | 8.1 × 103/μL | AST | 25 IU/L | P | 3.4 mg/dL |
| Lym | 14.0% | ALT | 21 IU/L | CRP | 4.21 mg/dL |
| RBC | 4.15 × 106/μL | γ‐GTP | 11 IU/L | TSH | 1.35 μIU/mL |
| Hb | 9.9 g/dL | ALP | 338 IU/L | fT4 | 1.04 ng/dL |
| Ht | 32.4% | LDH | 242 IU/L | fT3 | 2.7 pg/mL |
| MCV | 78.1 fl | CK | 38 IU/L | BNP | 85.0 pg/mL |
| Plt | 282 × 103/μL | TP | 7.2 g/dL | Urine | |
| ESR | 86 mm/h | Alb | 3.1 g/dL | TP/Cr | 0.14 g/gCr |
| Coagulation study | Cr | 0.58 mg/dL | |||
| APTT | 31.1 s | BUN | 18.2 mg/dL | ||
| PT‐INR | 0.980 | UA | 4.0 mg/dL | ||
| FDP | 8.7 μg/mL | Na | 139.4 mEq/L | ||
| D‐dimer | 5.8 μg/mL | K | 4.4 mEq/L | ||
| Fibrinogen | 397.2 mg/dL | Cl | 103.1 mEq/L | ||
Alb, albumin; ALT, alanine aminotransferase; ALP, alkaline phosphatase; APTT, activated partial thromboplastin time; AST, aspartate aminotransferase; BNP, B‐type natriuretic peptide; BUN, blood urea nitrogen; CK, creatine kinase; Cr, creatinine; CRP, C‐reactive protein; ESR, erythrocyte sedimentation rate; FDP, fibrin degradation product; fT3, free triiodothyronine; fT4, free thyroxine; γ‐GTP, gamma‐glutamyl transpeptidase; Hb, hemoglobin; Ht, hematocrit; LDH, lactate dehydrogenase; Lym, lymphocyte; MCV, mean corpuscular volume; Plt, platelet; PT‐INR, prothrombin time international normalized ratio; RBC, red blood cell; TP, total protein; TSH, thyroid‐stimulating hormone; UA, uric acid; WBC, white blood cell.
Figure 1.
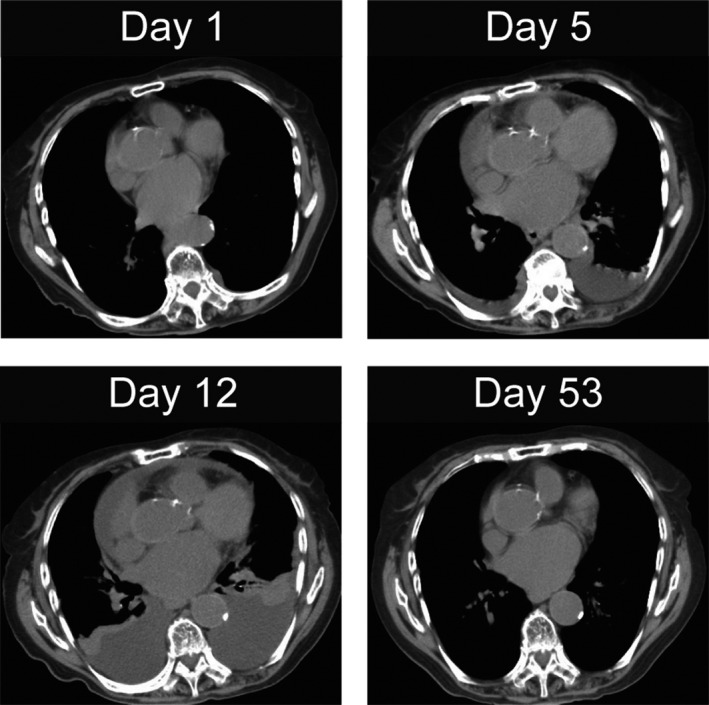
Chest computed tomography (CT) scan shows pleural and pericardial effusions on days 1, 5, 12, and 53
On day 5, her body temperature rose above 38°C and laboratory data showed an elevated CRP level (10.42 mg/dL) with mildly increased WBC (10 200/μL, lymphocyte: 13.0%). Physical examination did not reveal any obvious cause for the fever, and the CT scan did not demonstrate any possible source of inflammation; however, it revealed pleural and pericardial effusions (Figure 1, day 5). The pleural effusion was exudative according to the Light's criteria (pleural fluid protein/serum protein 0.57; pleural fluid LDH/serum LDH 1.29),4 and a Rivalta test was positive. The pH and adenosine deaminase levels of the pleural effusion were 7.2 and 26.5 IU/L, respectively. Bacterial cultures, including mycobacterial culture, of the pleural effusion were negative. Although we considered the possibility of recurrence of IVLBCL, pathological analysis of the pleural effusion (cell block section) did not show atypical cells. Random skin biopsy also failed to show atypical lymphocytes. The enhanced CT scan did not demonstrate any tumor mass, and tumor markers such as CEA, CA19‐9, ProGRP, and CYFRA 21‐1 were within the reference ranges. Therefore, we concluded that infection and malignancy were unlikely to be the cause of the pleural and pericardial effusions.
On day 12, percutaneous oxygen saturation (SpO2) markedly decreased to 91% under O2 4 L/min via nasal cannula and CRP was elevated to 13.73 mg/dL. Chest CT showed further accumulations of pleural and pericardial effusions (Figure 1, day 12). Laboratory examination revealed the following findings: antinuclear antibodies (ANA) positivity (×640 with a homogenous and speckled pattern) and anti–double‐strand DNA (dsDNA) antibodies above 400 IU/mL (off‐scale). She was negative for anti‐Smith (Sm), anti‐SSA/SSB, or anti‐U1 RNP antibodies. Serum complement (CH50) was below 14.0 U/mL (off‐scale), and lymphocyte count was low at 830/μL. Immunological test of the pleural effusion was also positive for ANA (×640 with a homogenous and speckled pattern). We suspected that pleuritis and pericarditis due to SLE caused her pleural and pericardial effusions. She was diagnosed with SLE based on the criteria of the Systemic Lupus International Collaborating Clinics (SLICC) classification criteria for SLE5: three clinical items (synovitis of PIP joints, serositis including pleuritis and pericarditis, and lymphocytopenia) and four immunological items (ANA positive, anti‐dsDNA antibody positive, low complement, and direct Coombs test positive in the absence of hemolytic anemia). The SLE Disease Activity Index (SLEDAI) score at diagnosis was 13.
Based on the diagnosis of SLE, we performed intravenous methylprednisolone pulse therapy (1000 mg per day for 3 days: from day 12 to day 14), followed by oral prednisolone (40 mg/d: equivalent to 1 mg/kg/d). Her respiratory status gradually improved (SpO2 > 97% in ambient air), and chest CT scan demonstrated decreased pleural and pericardial effusions on day 22, which disappeared almost completely by day 53 (Figure 1). CH50 was restored to 36.3 U/mL, and her initial complaints, bilateral lower extremity edema, and hand grip weakness also disappeared. After the dosage of prednisolone was tapered down, she was discharged on day 142.
3. DISCUSSION
Most autoimmune diseases commonly occur before old age. SLE is a typical example, which predominantly affects women with a high incidence during childbearing age and steep decline after menopause. The onset of SLE in patients over the age of 50 is uncommon, constituting 2%‐20% of all patients with SLE.6 Various researchers have examined differences between early‐onset SLE (20s, 30s, and 40s) and late‐onset SLE (over the age of 50), and the age at onset has been recognized as a factor associated with the clinical expression of SLE.2, 6, 7 Among several clinical manifestations of SLE, dermatological symptoms such as malar rash and photosensitivity are less frequent in late‐onset SLE patients (malar rash: 40.0% opposed to 73.3%, photosensitivity: 36.7% opposed to 76.6%).6, 7 Lupus nephritis is also less common (6.6% vs 46.6%).6 On the other hand, serositis and cytopenia are more frequently observed in late‐onset SLE patients.7, 8 Among laboratory manifestations of SLE, lower anti‐Sm antibody prevalence is one of the characteristic features of late‐onset SLE patients (10.5% vs 29.9%).2, 7 In our case, as well, the patient developed severe serositis, while no skin rash or kidney dysfunction was seen. Anti‐Sm antibody was not detected although ANA and anti‐dsDNA antibodies were strongly positive. In the course of aging, the immune system undergoes continual functional changes.9 For example, the number of circulating B cells decreases with age, and transcriptional changes in B cells lead to reduced ability to generate higher‐affinity antibodies.10 Although it is highly possible that age‐dependent changes and/or abnormalities of the immune system may be responsible for the characteristic features of late‐onset SLE patients, further investigation is necessary to uncover the mechanisms involving these features.
4. CONCLUSION
We herein report a case of very‐late‐onset SLE, in which serositis was the most drastic clinical manifestation. Patients with late‐onset SLE sometimes show atypical clinical features, which make it difficult to reach the correct diagnosis. Pleural effusion is commonly observed in several kinds of diseases, including heart failure, malignancy, and infection. In the primary care setting, it is critical to determine the initial strategy against rapid accumulation of pleural effusion, especially when it affects the patient's respiratory status. This case demonstrates that late‐onset SLE should be considered in the differential diagnosis of rapid accumulation of pleural effusion in the elderly.
In addition, investigation into late‐onset SLE has become much more important as aging of the general population continues. We hope this case presentation will add to the clinical knowledge about very‐late‐onset SLE and provide insight into autoimmune diseases in the elderly.
CONFLICT OF INTEREST
The authors have stated explicitly that there are no conflicts of interest in connection with this article.
Ikushima H, Mitsutake A, Hideyama T, et al. Severe pleuritis and pericarditis associated with very‐late‐onset systemic lupus erythematosus. J Gen Fam Med. 2018;19:53–56. https://doi.org/10.1002/jgf2.157
REFERENCES
- 1. Tsokos GC, Lo MS, Costa Reis P, Sullivan KE. New insights into the immunopathogenesis of systemic lupus erythematosus. Nat Rev Rheumatol. 2016;12:716–30. [DOI] [PubMed] [Google Scholar]
- 2. Lalani S, Pope J, de Leon F, Peschken C. Members of CaNIOS/1000 Faces of Lupus. Clinical features and prognosis of late‐onset systemic lupus erythematosus: results from the 1000 faces of lupus study. J Rheumatol. 2010;37:38–44. [DOI] [PubMed] [Google Scholar]
- 3. Arnaud L, Mathian A, Boddaert J, Amoura Z. Late‐onset systemic lupus erythematosus: epidemiology, diagnosis and treatment. Drugs Aging. 2012;29:181–9. [DOI] [PubMed] [Google Scholar]
- 4. Light RW, Macgregor MI, Luchsinger PC, Ball WC Jr. Pleural effusions: the diagnostic separation of transudates and exudates. Ann Intern Med. 1972;77:507–13. [DOI] [PubMed] [Google Scholar]
- 5. Petri M, Orbai AM, Alarcón GS, et al. Derivation and validation of the Systemic Lupus International Collaborating Clinics classification criteria for systemic lupus erythematosus. Arthritis Rheum. 2012;64:2677–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tomic‐Lucic A, Petrovic R, Radak‐Perovic M, et al. Late‐onset systemic lupus erythematosus: clinical features, course, and prognosis. Clin Rheumatol. 2013;32:1053–8. [DOI] [PubMed] [Google Scholar]
- 7. Ramos‐Casals M, García‐Carrasco M, Brito MP, López‐Soto A, Font J. Autoimmunity and geriatrics: clinical significance of autoimmune manifestations in the elderly. Lupus. 2003;12:341–55. [DOI] [PubMed] [Google Scholar]
- 8. Koh ET, Boey ML. Late onset lupus: a clinical and immunological study in a predominantly Chinese population. J Rheumatol. 1994;21:1463–7. [PubMed] [Google Scholar]
- 9. Pinti M, Appay V, Campisi J, et al. Aging of the immune system: focus on inflammation and vaccination. Eur J Immunol. 2016;46:2286–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Khurana S, Frasca D, Blomberg B, Golding H. AID activity in B cells strongly correlates with polyclonal antibody affinity maturation in‐vivo following pandemic 2009‐H1N1 vaccination in humans. PLoS Pathog. 2012;8:e1002920. [DOI] [PMC free article] [PubMed] [Google Scholar]


