Figure 1.
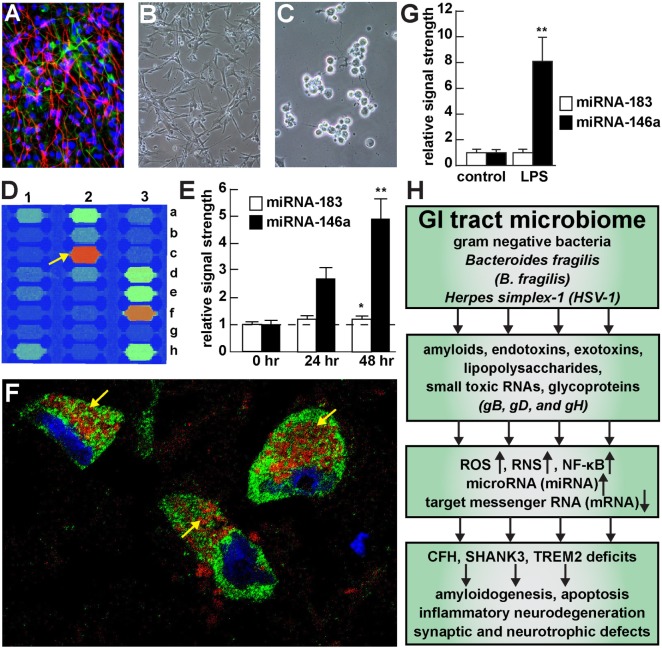
Viral and bacterial contribution to neuroinflammatory signaling in sporadic Alzheimer’s disease (AD) brain—(A) primary human neuronal-glial (HNG) cells after ~2 weeks in primary coculture; the cell density is approximately 75% neurons and 25% astroglia at ~60% confluency; human primary neuronal and glial “support” cell cocultures are utilized because human neuronal cells do not culture well by themselves (11); neuronal cells are stained with neuron-specific β-tubulin (red; λmax = 690 nm), glial cells are stained with glial-specific glial fibrillary acidic protein (GFAP; green; λmax = 525 nm), and nuclei are stained with DAPI/Hoechst 33258 stain (blue; λmax = 470 nm); photo magnification 30×; (B) control HNG cells in primary culture; as in previous panel; light microscopy 30×; (C) HNG cells exposed to HSV-1 for 48 h; note “rounding up of cell bodies and retraction of neurites”; (D) miRNA array analysis of an overexpressed proinflammatory miRNA-146a in HSV-1 treated HNG cells; on this miRNA array an upregulated miRNA-146a is indicated at position 2c (33); (E) time course of induction (0, 24, and 48 h) of a pathogenic miRNA-146a in HNG cells by HSV-1; at 48 h the abundance of miRNA-146a was almost fivefold over controls; miRNA-183 represents an unchanging miRNA control; (F) association of lipopolysaccharide (LPS) with the neuronal cytoplasm and the periphery of neuronal nuclei in AD neocortex—NeuN (neuron-specific green stain; λmax = 520 nm), LPS (red stain; λmax = 690 nm), and DAPI (blue stain; λmax = 470 nm); human superior temporal lobe AD neocortex (Brodmann A22); note organization of LPS into a “clathrin-like” lattice or “net” within the neuronal cell cytoplasm (yellow arrows); (G) induction of the proinflammatory miRNA-146a in LPS-stressed HNG cells is almost eightfold over controls after 48 h; (H) flow chart of the potential contribution of the GI tract-resident microbiome-abundant Gram-negative bacilli Bacteroides fragilis and herpes simplex-1 (HSV-1) to neuropathological pathways; stressed B. fragilis secrete a remarkable quantity of neurotoxins that include amyloids, endotoxins, exotoxins, LPS, and small toxic microRNA-like RNAs; upon HSV-1 invasion of human neurons cell surface proteins serve as receptors for viral entry and the HSV-1 glycoproteins gB, gD, and gH are required for infection of, and the maintenance of latency in human neurons; in both cases B. fragilis and HSV-1 in turn induce the evolution of reactive oxygen species (ROS), reactive nitrogen species (RNS), and the proinflammatory transcription factor NF-κB and upregulate a small family of NF-κB-sensitive microRNAs (miRNAs) including miRNA-146a that interact with the 3′-untranslated region (3′-UTR) of messenger RNA (mRNA) targets (1, 36, 37). In the case of B. fragilis signaling this drives the downregulation of complement factor H (CFH), the postsynaptic cytoskeletal SHANK3 and the triggering receptor expressed in myeloid/microglial cells TREM2; deficits in CFH, SHANK3, and TREM2 resulting in the inability to clear excessive amyloid from brain cells, amyloidogenesis, apoptosis, inflammatory neurodegeneration and synaptic and neurotropic deficits. Because all miRNA fractions were obtained from short postmortem interval (PMI) human tissue samples and miRNAs have been reported to have a high rate of depolymerization (degradation) under physiological, and especially pathophysiological conditions, only upregulated miRNAs were studied here; in fact miRNA upregulation and mRNA downregulation appears to be a very common posttranslational genetic regulatory mechanism in the human and murine CNS [see text (38, 39)]. Note that parts of Figure 1 have been considerably updated from a previous version (37, 40–42).