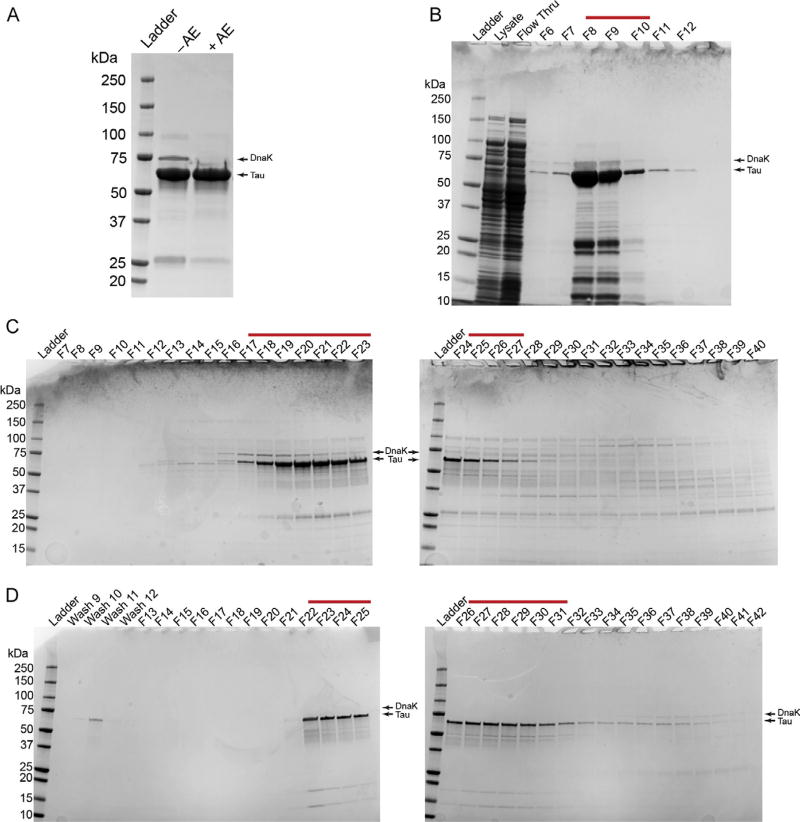
FIG. 1.
The SDS-PAGE and Coomassie gel staining analysis of a typical purification of recombinant human tau protein. (A) A final preparation of tau without the anion exchange cleanup step (−AE) is compared to a preparation of tau with anion exchange (+AE). Note the clear removal of DnaK (i.e., ~70kDa) when the protein preparation is cleaned using the anion exchange procedure. 10µg purified protein was loaded per lane. (B) A gel showing the bacterial lysate, the column flow through from the sample application (Flow Thru), and elution fractions 6–12 (F6–12) from the Talon column His-tag purification step. Fractions 8–10 were collected for further purification (red line). (C) Two gels showing fractions 7–40 (F7–40) of the S500 column size exclusion chromatography step. Fractions 17–27 were collected for further purification (red line). (D) Two gels showing some of the wash fractions and fractions 13–42 from the anion exchange column cleanup step (F13–42). It is important to avoid fractions containing DnaK at this step; fractions 22–31 were collected for further purification (red line). This is a typical preparation of full-length wild-type human tau (hT40, 2N4R, 441 amino acids+C-terminal 6× His-tag). Note that the tau and DnaK bands are marked by arrows in each panel.