Abstract
Heart failure (HF) secondary to myocardial infarction (MI) is linked to kidney complications that comprise cellular, structural, functional, and survival indicators. However, HF research is focused on left ventricular (LV) pathology. Here, we determined comprehensive functional analysis of the LV using echocardiography in transition from acute heart failure (AHF) to progressive chronic heart failure (CHF) pathology and developed a histological compendium of the cardiosplenic and cardiorenal networks in pathological remodeling. In surgically induced MI using permanent coronary ligation, the LV dysfunction is pronounced, with myocardium necrosis, wall thinning, and 20–30% LV rupture events that indicated AHF and CHF pathological remodeling in C57BL/6 male mice (2–4 mo old, n = 50). Temporal LV function analysis indicated that fractional shortening and strain are reduced from day 1 to day 5 in AHF and sustained to advance to CHF from day 28 to day 56 compared with naïve control mice (n = 6). During the transition of AHF (day 1 to day 5) to advanced CHF (day 28 to day 56), histological and cellular changes in the spleen were definite, with bimodal inflammatory responses in kidney inflammatory biomarkers. Likewise, there was a unidirectional, progressive, and irreversible deposition of compact collagen in the LV along with dynamic changes in the cardiosplenic and cardiorenal networks post-MI. The renal histology and injury markers suggested that cardiac injury triggers irreversible dysregulation that actively alters the cardiosplenic and cardiorenal networks. In summary, the novel strategies or pathways that modulate comprehensive cardiosplenic and cardiorenal networks in AHF and CHF would be effective approaches to study either cardiac repair or cardiac pathology.
NEW & NOTEWORTHY The present compendium shows irreversible ventricular dysfunction as assessed by temporal echocardiography while histological and structural measurements of the spleen and kidney added a novel direction to study cardiosplenic and cardiorenal networks in heart failure pathology. Therefore, the consideration of systems biology and integrative approach is essential to develop novel treatments.
Listen to this article's corresponding podcast at http://ajpheart.podbean.com/e/temporal-dynamics-of-acute-and-chronic-heart-failure/.
Keywords: fibrosis, ischemia injury, temporal echocardiography
INTRODUCTION
Heart failure (HF) secondary to ischemic injury is a global epidemic leading to advanced chronic HF (CHF) pathology, particularly in the aged. Almost 6 million people in the United States and >23 million worldwide are suffering from this end-stage disease that costs 1–2% of the healthcare expenditure in Europe and North America (1, 26, 28). Cellular or molecular recovery or reduction of infarct size-mediated reverse remodeling does not always equate with comprehensive functional or survival outcome benefits (13). Therefore, to develop novel treatment strategies, it is of paramount importance to understand integrative or comprehensive approaches to offer new mechanism(s) of cardioprotection or cardiac pathology. In this report, we present comprehensive and dynamic changes seen in acute HF (AHF) and CHF pathology, with major emphasis on cardiosplenic and cardiorenal networks after myocardial infarction (MI).
One obvious question is why to study the spleen and renal integrative and comprehensive networks in cardiac pathology or cardiac repair? The spleen is a highly organized lymphoid reservoir that provides leukocytes for clearance in response to myocardial injury, and it contributes to cardiac protection or pathology (15, 34). The spleen’s innate and adaptive mechanisms function in a unique and sequential manner; therefore, splenic changes in AHF and CHF pathology are important for myocardial pathology and cellular recovery (7, 15, 23, 34). Until recently, study of the MI-induced pathological remodeling process centered on the myocardium in the acute setting; now, the concept of exclusive myocardium recovery has been shown to be incorrect because of comedication, comorbidity, and heterogeneity of advanced HF disease pathology in the clinical setting (13).
MI-induced acute and chronic pathological remodeling is unidirectional and irreversible and equates to dysregulation in the splenic and cardiorenal networks (10). Here, we provide a compendium of comprehensive temporal LV functional analysis with dynamic structural changes in the left ventricle (LV), spleen, and kidney during the transition from AHF to CHF pathology. The presented report comprises three comprehensive and integrative networks because 1) the splenic reservoir supplies immune cells to the infarcted LV for healing, 2) a MI develops progressive LV dysfunction with pronounced structural changes in AHF and CHF pathology, and 3) the postinfarction myocardial injury develops feedforward progressive signaling to trigger renal structural pathology. Thus, the present compendium of cardiosplenic and cardiorenal networks in AHF and advanced ischemic CHF pathology is a novel and useful tool to investigate cardioprotection or pathobiology in the HF setting.
MATERIALS AND METHODS
Animal compliance.
All surgery protocols involving animals conformed with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (Revised 2015) and were approved by the Animal Care and Use Committee of the University of Alabama at Birmingham.
Coronary artery ligation surgery to induce MI.
This study comprised a total of 56 male C57BL/6 mice weighing 22–25 g. A group of 50 mice underwent coronary ligation surgery (1 mm distal to the left atrium) by the open-chest permanent coronary ligation procedure, particularly ligation of the left anterior descending coronary artery (LAD) occlusion without a reperfusion protocol, and 6 mice were maintained as naïve controls. Reperfusion was not included, since the objective was to study CHF pathology. A set of 7–10 mice was euthanized for each time point with day 1 and day 5 as acute time points and day 25 and day 56 as chronic time points (10, 20, 21). Mice that died within 24 h were counted as perioperative mortality, whereas those that died 24 h were evaluated for the causes of death, either rupture or congestive HF.
Transthoracic echocardiography.
Echocardiography images of C57BL/6 male mice were acquired using a VisualSonics Vevo 3100. The non-MI surgery group was designated as the baseline no-MI day 0 naïve control, and further post-MI day 1 through day 56 images were acquired for temporal measurements. Mice were briefly anesthetized using isoflurane in a closed chamber before being placed on a temperature-maintained platform in the supine position. The paws were taped over the electrode pads, which were lubricated with electrode Crème (part no. 600-0001-01-S, Indus Instruments). After the mouse was secured on the platform, the ECG signal was monitored through the display screen to ensure the placement and physiological parameter monitoring were correct. Once the mouse was secure, the platform was then turned to the left frontmost position to access the LV for a parasternal long-axis view. Temperature-controlled ultrasound gel was then applied to the chest above the LV area, and the probe (MX400, axial resolution: 50 μm) was lowered on the mouse at an 11° angle to the sternum. Once in place, the B-mode option was selected on the Vevo3100 interface, and the screen showed the image from the probe. As the probe was lowered and adjusted on the LV, the operator focused on the screen and made adjustments so that the aorta lined up with the apex in the parasternal long axis. For the optimal view, three consecutive images were captured at heart rates > 450 beats/min, the M-mode was selected, and the cursor was then placed across the LV so that the papillary was slightly in view and the LV walls were visible. Three consecutive images were captured for this mode as well. LV parasternal long-axis B-mode images were used in circumferential strain analysis, and M-mode images were used with the LV wall trace tool to measure wall thickness in the LV. Next, the probe was repositioned for the short-axis view by raising the probe and turning it perpendicular to the sternum. For B-mode and M-mode, three consecutive images were acquired at heart rates above 450 beats/min, and B-mode images were used for radial strain analysis and M-mode images were used for wall thickness.
Echocardiography M-mode image analysis.
For wall thickness and functional parameter analysis, a short-axis M-mode image was selected where the walls were clearly visible throughout diastole and systole; the heart rate was between 450 and 550 beats/min. The measurement tools were opened to choose the LV wall trace icon, and, using the image, points at systole and diastole were clicked on the posterior and anterior portions of the walls. The LV wall trace provides LV diameters and volumes and calculates fractional shortening and ejection fraction. For wall thickness measurements, the same image was used. For diastole, the interventricular septal end-diastolic measurement was selected and the septal part of the diastole was selected and then dragged to the posterior wall and selected to obtain the interventricular septal end-diastolice and LV posterior wall. LV internal dimension was listed; however, the measurements obtained from the LV wall trace performed earlier were used for this parameter. This was repeated for the systole wall thicknesses by selecting interventricular septal end-systole and repeating the previously listed steps. For strain analysis, the long-axis B-mode image was opened with strain options. At the beat selection screen that is shown next, a straight line was drawn through the LV near the papillary muscle and three concurrent beats that do not have an interruption from breathing were selected. After the beats had been selected, LV wall trace was selected, the video was paused at systole, and the LV area was traced, stopping just before the aorta on both sides. Adjustments to endocardial area were made as necessary, and analyze was selected to receive strain information (3, 21).
Necropsy and LV, spleen, and kidney harvest.
No-MI control day (day 0) and day 1, 5, 28, and 56 mice were anesthetized with isoflurane briefly as previously reported. The kidney, lungs, LV, and right ventricle were separated and weighed individually. The LV was divided into the apex (infarcted area), midcavity, and base (remote area) under a microscope. The kidneys and LV were collected and weighed. One-half part of the right kidney and a middle section of the left kidney were either fixed in 10% zinc formalin for immunohistochemistry or kept in OCT for cryosectioning. The rest of the kidney and LV was snap frozen for molecular analysis as previously described (11, 17, 20).
LV and spleen hematoxylin and eosin staining.
For histological measurements, LV transverse and spleen longitudinal sections were embedded in paraffin and sectioned. Sections were stained with hematoxylin and eosin, and LV images were acquired for each mouse. With the use of a microscope (BX43) with an attached camera (Olympus DP73), a total of five to seven images were acquired for the LV infarct area, including the border zone and remote zone. Hematoxylin and eosin-stained images were graded on the scale from 0 to 10 by an inflammatory response where 0 indicates a normal LV, 3–5 is moderate, and 5–10 is severe. A total of five to six images were analyzed per mouse per time point (6).
Picrosirius red staining.
For picrosirius red staining, paraffin-embedded unstained sections of LV tissue were deparaffinized in citrisolv (Fisher Scientific) and rehydrated through subsequent washes of ethanol. The picrosirius red staining was done and quantified as previously described (11, 17, 21).
Periodic acid-Schiff staining.
For no-MI and post-MI conditions (days 1−56), the kidneys were collected and weighed. The longitudinal middle slice of the kidney, taken through the hilum, was embedded in paraffin, stained with periodic acid-Schiff reagent, and scored as previously described (10).
Confocal microscopy.
For immunofluorescence, spleen and kidney cryosections were fixed, permeated and blocked, and then incubated with antibody against F4/80 (ab6640, Abcam)/Foxp3 (14-5773-82, eBioscience) combination and nephrin and neutrophil gelatinase-associated lipocalin (NGAL) overnight. Next, sections were incubated with secondary antibody conjugated with Alexa 488 (A11008, Molecular Probes) and Alexa 555 (A21422, Molecular Probes) for 1 h. Nuclei were stained with Hoechst. Confocal microscopy was performed on a Nikon A1 high-resolution microscope, and stack images were acquired according to standard protocols. Images were representative of four to five sections for two mice per group. Foxp3+ cells, three to four sections per slide for each time point, were quantified using Image software.
Real-time PCR.
Kidney RNA was isolated from non-MI control day (day 0) and post-MI (day 1–56) time points using the TRIzol method. For quantitative PCR, reverse transcription was performed with 2.5 μg total RNA using the SuperScript VILO cDNA Synthesis Kit (Invitrogen). Quantitative PCR for NGAL was performed using TaqMan probes (Applied Biosystems) on master cycler ABI, 7900HT. Gene levels were normalized to hypoxanthine phosphoribosyltransferase-1 as the housekeeping control gene. Results were reported as 2−ΔCt (ΔΔCt) values (where Ct is threshold cycle). All experiments were performed in duplicate with n = 6 mice/group.
Plasma creatinine.
Plasma creatinine levels for no-MI day 0 naïve controls and post-MI days 1, 5, 28, and 56 were determined using LC-MS/MS as previously described (10).
NGAL ELISA.
The NGAL or lipocalin-2 mouse simple-step ELISA kit (ab199083, Abcam) was used to determine plasma NGAL levels according to the manufacturer’s instruction.
Statistical analysis.
Data are presented as means ± SE. Multiple time point comparisons were performed using one-way ANOVA followed by the Student-Newman-Keuls posttest. The post-MI survival rate was analyzed by Kaplan-Meier survival analysis and compared by the log-rank test. Values of P < 0.05 were considered statistically significant.
RESULTS
Permanent coronary ligation drives time-dependent model of AHF and advanced CHF.
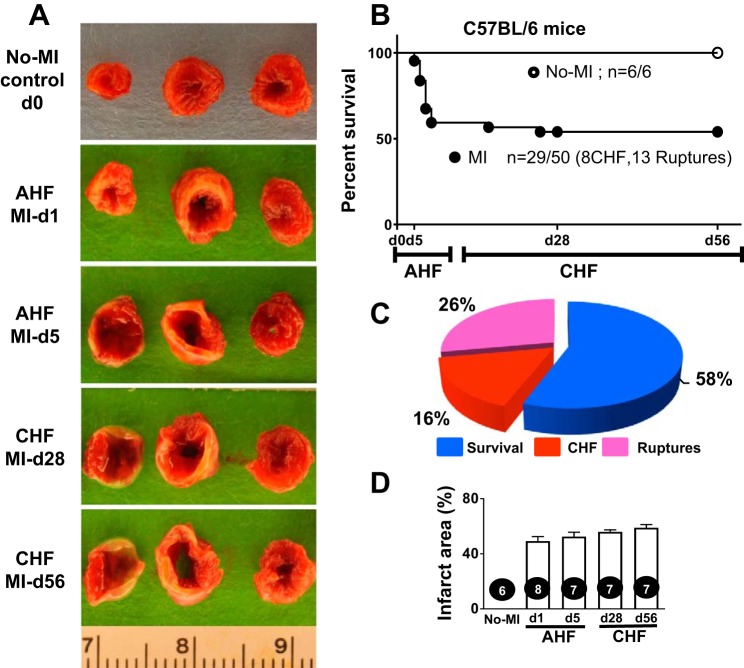
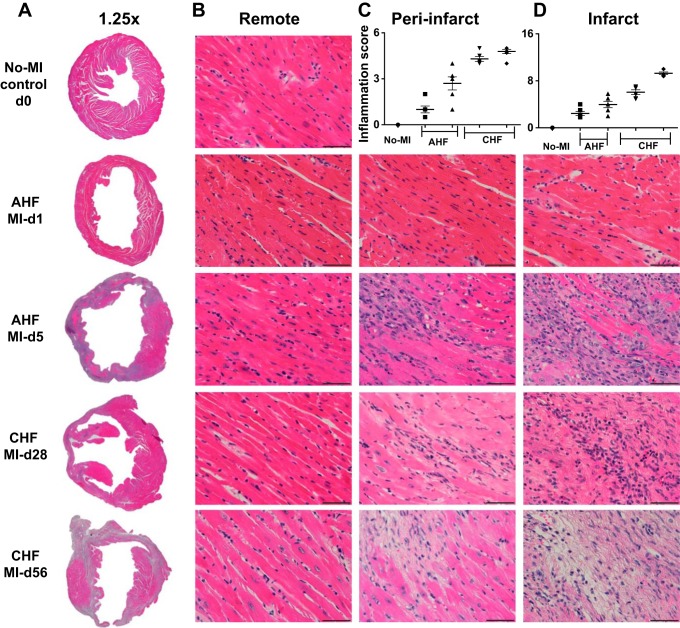
In response to successful MI surgery, all survivor mice develop advanced CHF. Nearly 42% of mice died with 2–7 days post-MI because of either LV rupture or congestive HF, which is indicative of the severity of the LAD ligation model to study AHF and CHF pathology (Fig. 1). Of note, both the ligation distance from the left atrium and mouse strain are critical for post-MI dilation and survival analysis. The pie chart in Fig. 1 shows that 26% of the mice had LV rupture (13 of 50 mice) and 16% of the mice had CHF (8 of 50 mice) post-MI. After MI, the infarcted areas were consistent in size as shown using 2,3,5-triphenyltetrazolium chloride with sustained LV dilative images that indicated prolonged ischemia-mediated cardiac remodeling toward CHF pathology (Fig. 1 and Table 1). The infarcted area represents the magnitude of myocardial injury in AHF and CHF pathology. In murine hearts, cardiac myocytes represent 58% of the myocardium (40); however, after MI, the transmural infarcted area was replaced 100% with the stable extracellular matrix, which indicates a sign of fibrotic and irreversible pathological remodeling in advanced CHF pathology compared with the naïve heart. Hematoxylin and eosin staining of myocardial samples from days 1, 5, 28, or 56 post-MI showed signs of progressive end-stage HF with an increase in the size of cardiomyocytes in the remote area, wall thinning of the infarcted area, and LV dilation and scar formation from day 5 onward to day 56 of CHF pathology. The progressive wall thinning in AHF pathology is linked to LV dysfunction and loss of nearly all cardiomyocytes as a result of apoptosis and necrosis in the infarcted area in the advanced CHF pathology post-MI (Fig. 2). Thus, 2,3,5 triphenyltetrazolium chloride-stained LV morphological and histological results showed an irreversible pathological remodeling with extensive changes in the remote peri-infarcted area (commonly called the area at risk or border zone); LV cardiomyocytes that are replaced with fibrotic scar progressively advance to CHF pathology post-MI.
Fig. 1.
Temporal dynamics of survival and left ventricular (LV) infarcted area in acute heart failure (AHF) and chronic heart failure (CHF) pathology after myocardial infarction (MI). A: LV representative images stained with 2,3,5-triphenyltetrazolium chloride indicated the infarct area (white) in AHF and CHF pathology. B: survival curve of C57BL/6 mice post-MI analyzed using a Kaplan-Meier log-rank test showing 58% survival in heart failure (HF) pathology with no death in naïve control mice. C: pie chart indicating 58% survival (29 of 50 mice), 26% mortality resulting from rupture (13 of 50 mice), and 16% mortality resulting from CHF (8 of 50 mice). n = 56 mice total for AHF and CHF temporal dynamic measurements.
Table 1.
Necropsy parameters in AHF and CHF pathology of C57BL/6J post-MI
| Necropsy Parameters | Naïve Controls (Day 0) | AHF MI Day 1 | AHF MI Day 5 | CHF MI Day 28 | CHF MI Day 56 |
|---|---|---|---|---|---|
| n | 6 | 8 | 7 | 7 | 7 |
| Body wt, g | 22.5 ± 1.31 | 25.8 ± 1.5 | 20.6 ± 0.57 | 25.7 ± 0.6 | 30.2 ± 0.5 |
| LV, mg | 72.8 ± 2.19 | 87.4 ± 4.4* | 96.0 ± 2.84* | 111.0 ± 4.1* | 112.8 ± 2.7* |
| LV/body wt, mg/g | 3.3 ± 0.15 | 3.4 ± 0.1 | 4.7 ± 0.10* | 4.3 ± 0.2* | 3.7 ± 0.1* |
| RV, mg | 17.2 ± 0.60 | 17.8 ± 1.1 | 20.6 ± 0.76* | 24.3 ± 0.8* | 24.7 ± 1.1* |
| Spleen, mg | 60.5 ± 4 | 56.4 ± 4 | 83.1 ± 8 | 73.4 ± 2.4* | 95 ± 8.4* |
| Right kidney, mg | 132 ± 6 | 146 ± 9 | 122 ± 6 | 154 ± 6* | 153 ± 6* |
| Left kidney, mg | 148 ± 12 | 152 ± 9 | 129 ± 8 | 155 ± 6 | 145 ± 7 |
| RV mass/body wt | 0.8 ± 0.03 | 0.7 ± 0.1 | 1.0 ± 0.04* | 0.9 ± 0.04* | 0.8 ± 0.04* |
| Lung mass/body wt, mg/g | 7.6 ± 0.93 | 6.2 ± 0.71 | 8.8 ± 0.51 | 5.5 ± 0.10 | 5.2 ± 0.20 |
| Tibia, mm | 16.9 ± 0.14 | 16.8 ± 0.12 | 16.4 ± 0.10 | 16.7 ± 0.10 | 17.5 ± 0.12 |
Values are means ± SE; n, sample size. AHF, acute heart failure; CHF, chronic heart failure; MI, myocardial infarction; LV, left ventricle; RV, right ventricle.
P < 0.05 vs. naïve controls.
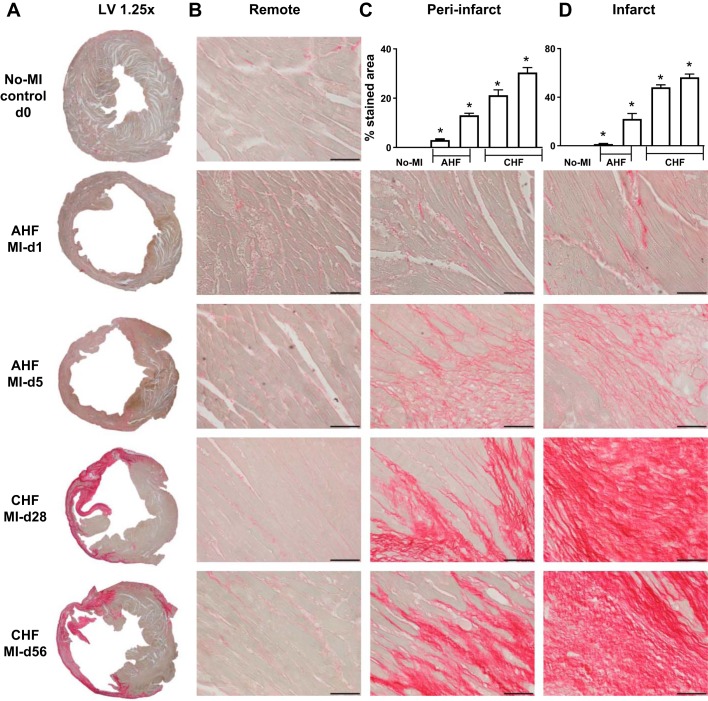
Fig. 2.
Time-dependent structural changes in the infarct, peri-infarct (border zone or area at risk), and remote (noninfarcted) areas in acute heart failure (AHF) and chronic heart failure (CHF) pathology. A−C: hematoxylin and eosin (H&E) staining of ×1.25 images of the complete left ventricle (LV; A), ×40 images of the remote zone (B), and ×40 images of the peri-infarct zone and quantitative analyses as a bar graph (C). D: ×40 images of the infarct zone in C57BL/6 mice during AHF and CHF displaying changes in ultrastructure and quantitative analyses as a bar graph. n = 7–8 mice/group. Scale bar = 50 μm.
Heart dysfunction is definite and irreversible in AHF and CHF pathology post-MI.
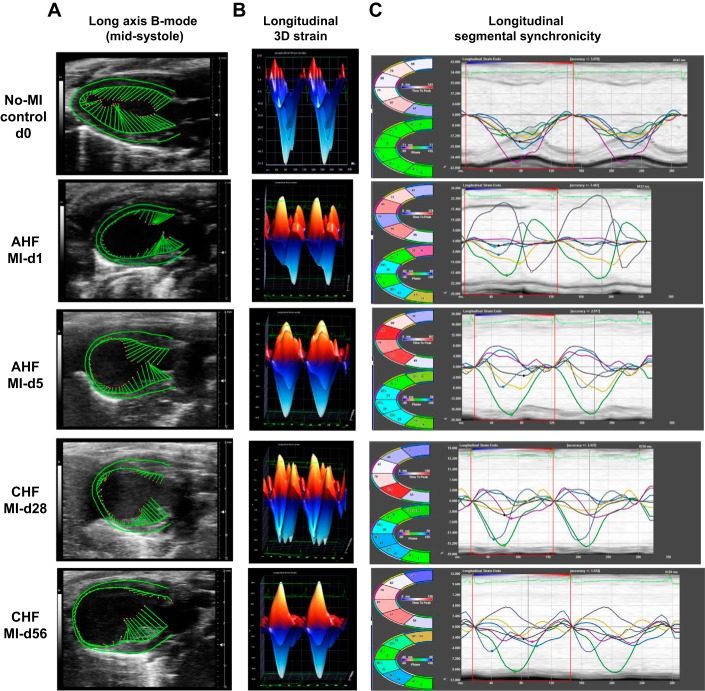
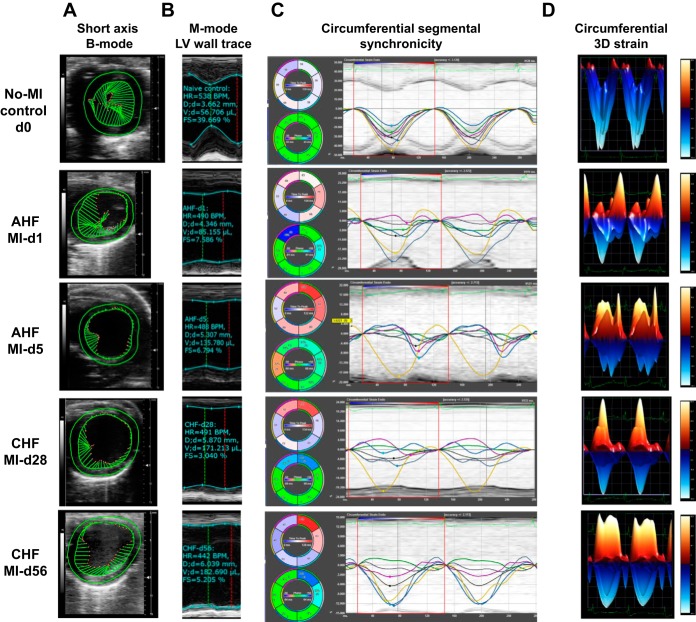
In response to myocardial injury, cardiac remodeling is the fundamental feature that occurs in the progression of advanced CHF, as characterized by changes in shape, size, and function of the myocardium post-MI. This dynamic change in LV function is marked by a significant increase of LV dimensions (end-diastolic dimension and end-systolic dimension), volumes (end-diastolic volume and end-systolic volume), and reduced strains (circumferential and longitudinal), with marked segmental dyssynchrony (Fig. 3, A–C and Table 2). LV trace and speckle tracking-based strain analysis showed that there was immediate LV dysfunction within 24 h: fractional shortening was reduced from the standard range of 35–40% to 8–10% at MI day 1 and, furthermore, there was a sustained reduction of contractility to 6–7% at MI day 5 that indicated AHF. The transition of AHF to CHF was marked by the sustained progression of LV dysfunction, showing 4.7 ± 0.0.04% (Table 2) fractional shortening at MI day 28 and 5.2 ± 0.04% at MI day 56, an irreversible pattern of LV function that indicates CHF. In the CHF setting, there were limited changes in fractional shortening from day 28 to day 56 post-MI, and the dilation (end-diastolic dimension) continually increased from 3.75 ± 0.74 mm at baseline to 6.06 ± 1.58 mm through MI day 56 (Table 2). The sustained LV dilative dysfunction until day 5 post-MI indicates AHF pathology. In the chronic phase of myocardial healing from day 28 to day 56, there was sustained wall thinning and dilatation that indicated signs of CHF post-MI.
Fig. 3.
Temporal long-axis B-mode longitudinal strain (two- and three-dimensional) and segmental strain indicated the progressive dynamic changes in left ventricular (LV) size, shape, and function in acute heart failure (AHF) and chronic heart failure (CHF) after myocardial infarction (MI). A: echocardiographic representation of speckle-tracking analysis in the long-axis B-mode; the LV is in midsystole. B and C: longitudinal three-dimensional strain (B) and longitudinal segmental synchronicity (C) of the LV. Images are representatives of n = 7–8 mice/group.
Table 2.
Echocardiography measurements in AHF and CHF pathology of C57BL/6J post-MI
| Parameters/ Groups | Naïve Controls (Day 0) | AHF MI Day 1 | AHF MI Day 5 | CHF MI Day 28 | CHF MI Day 56 |
|---|---|---|---|---|---|
| n | 6 | 8 | 7 | 7 | 7 |
| Heart rate, beats/min | 471 ± 129 | 481 ± 171 | 481 ± 187 | 457 ± 85 | 487 ± 122 |
| EDD, mm | 3.75 ± 0.74 | 4.63 ± 0.99* | 5.33 ± 1.62* | 6.04 ± 1.74* | 6.06 ± 1.58* |
| ESD, mm | 2.27 ± 0.68 | 4.26 ± 1.18* | 5.00 ± 1.69* | 5.76 ± 1.81* | 5.74 ± 1.43* |
| Fractional shortening % |
39.5 ± 0.08 | 8.0 ± 0.08* | 6.0 ± 0.07* | 4.7 ± 0.04* | 5.2 ± 0.04* |
| IVSd, mm | 0.71 ± 0.34 | 0.45 ± 0.39* | 0.40 ± 0.39* | 0.37 ± 0.24* | 0.29 ± 0.21* |
| PWTd, mm | 0.67 ± 0.37 | 0.49 ± 0.23* | 0.45 ± 0.27* | 0.36 ± 0.17* | 0.31 ± 0.31* |
| IVSs, mm | 1.10 ± 0.24 | 0.54 ± 0.46* | 0.46 ± 0.43* | 0.45 ± 0.24* | 0.36 ± 0.42* |
| PWTs, mm | 1.09 ± 0.26 | 0.54 ± 0.34* | 0.49 ± 0.30* | 0.41 ± 0.22* | 0.36 ± 0.29* |
| GLS | −20.19 ± 12.51 | −4.05 ± 5.00* | −2.68 ± 6.83* | −3.90 ± 5.46* | −3.74 ± 3.28* |
| GCS | −32.41 ± 13.59 | −7.67 ± 14.42* | −3.37 ± 6.71* | −4.33 ± 8.67* | −7.31 ± 9.30* |
Values are means ± SE; n, sample size. AHF, acute heart failure; CHF, chronic heart failure; MI, myocardial infarction; EDD, end-diastolic dimension; ESD, end-systolic dimension; PWTd, posterior wall thickness at diastole; IVSd, interventricular septal end diastole; IVSs, interventricular septal end systole; PWTs, posterior wall thickness at systole; GLS, global longitudinal strain; GCS, global circumferential strain.
P < 0.05 vs. naïve controls at the respective time point.
The pattern of segmental strain is divided into six sections in Figs. 3C and 4C and goes from all areas represented in green (time to peak strain is <10% out of synchrony from the time to peak strain from the average of all 6 sections) to an immediate effect in the infarcted area sections falling out of synchrony from immobility that increases strain toward zero (reduced contractility) from the myocardium. The functioning remote areas (near the base) initially showed some retention of synchrony as they began to compensate for the inactive infarct area during AHF. Longitudinal strain is also divided in the following six sections in Fig. 3C: posterior base (green), posterior mid (white), posterior apex (light blue), anterior base (dark blue), anterior mid (yellow), and anterior apex (magenta). Circumferential strain synchronicity segregates a total of six LV regional measures in Fig. 4C: anterior free wall (green), lateral wall (white), posterior wall (cyan), inferior free wall (blue), posterior septal wall (yellow), and anterior septal wall (magenta). These sections are plotted as curvilinear data for each region tracked. The black line indicates the average global function in the curvilinear form, showing synchrony in clear peaks and valleys in controls but sustained regional dyssynchrony of infarcted sections in AHF and functional regions in CHF. In the progression from AHF to CHF, the strain was decreased in the functional areas of the LV, the posterior and anterior base for longitudinal and inferior free wall and posterior septal wall for circumferential, as they worked to compensate for the immobile infarcted areas. Synchronicity changed in the infarcted areas (the posterior/anterior apex for longitudinal and anterior free wall/lateral wall for circumferential) during CHF, as the LV normalized to its new postinfarcted size, shape, and remodeled rhythm (Fig. 4, A–D). The functioning remote sections compensate with larger contractility (negative strain) during the progression from AHF to CHF, leading to a minimal decrease in the global longitudinal strain (GLS) and global circumferential strain (GCS) during CHF. This occurs as the infarcted sections become more in phase as time progresses and the functional areas become less synchronized (larger gap in time to peak strain from average) with the immobilized infarcted regions. With progressive LV size dilation, the LV volume increased, and LV dysfunction continued to become more obvious with a change in shape from an elliptical pattern to a circular pattern that reached GLS closer to zero at the peak of AHF pathology. As the heart adjusts to the remodeled post-MI state, strain and synchronicity stabilize through the progression of CHF pathology. Transition of strain from higher negative numbers to positive or closer to zero indicated a greater loss of contractility that peaked at the end of AHF (MI day 5, −2.68 ± 6.83 GLS); as the heart progressed through AHF to CHF, there was slight decrease of strain because of increased contractility of the functional remote areas as the MI-compensated heart rhythm normalized to survive its new postinjury form. The main feature of strain analysis software is how it uniquely allows the visualization of the specific LV areas (infarcted or noninfarcted) that show signs of dysfunction and dyssynchrony, which was unfeasible using the traditional systolic markers of fractional shortening (short-axis M-mode) and ejection fraction (long-axis B mode). In the progression of AHF, the GLS moved closer to zero (−2.68 ± 6.83, MI day 5), although contractility slightly improved (more negative strain) in the functional LV areas in the progression from AHF to CHF (−3.74 ± 3.28, MI day 56). As the infarcted heart adjusted to the post-MI remodeled state from the most severe point of AHF at day 1 to day 5 post-MI, synchrony improved in infarcted regions (now closer to the average time to peak) and lessened in functional areas. To maintain consistent outcomes from AHF to CHF, samples with an infarcted area of <40% were excluded from the functional and histological analyses. Presented speckle tracking-based analyses are useful to eliminate intraobserver and interobserver variations because differences in image acquisition may contribute to the variation of heart functional outcome from mouse to mouse or laboratory to laboratory. The infarcted myocardial deformation and sustained progressive dysfunction can be distinguished by the result of the six segmental areas that are used to calculate the overall GLS and GCS strain post-MI. Thus, an MI-induced complex heart functional pattern marked by myocardial deformation that shows thinning at the apex and compensatory thickening at the basal anterior and basal inferior indicated heart dysfunction, loss of strain, and dyssynchrony in AHF and CHF pathological remodeling.
Fig. 4.
Consecutive short-axis B-mode, left ventricular (LV) wall trace M-mode, and segmental strain indicated the irreversible cardiac remodeling in acute heart failure (AHF) and chronic heart failure (CHF) pathology after myocardial infarction (MI). A: echocardiographic representation of speckle-tracking analysis in short-axis B-mode equipped with M-mode wall trace. B: representative short-axis M-mode LV wall trace. C: representative echocardiographic traces of short-axis circumferential segmental synchronicity. D: circumferential three-dimensional strain. LV images are representative of n = 7–8 mice/group.
Advanced CHF is marked with fibrotic remodeling in ischemic pathology post-MI.
Scar formation is a unique compensatory mechanism of infarcted LV wound healing that is marked by fibroblast slippage, lengthening, and activated secretory capacity to produce collagen (29). Fibrotic scars were the result of prolonged ischemia that led to nearly 100% replacement of myocytes with matured compact collagen matrix in the infarcted area and patchy fibrosis in the peri-infarct area. The remote area showed limited fibrotic remodeling during the transition of AHF to CHF pathology (Fig. 5, A−D). Post-MI, the scar formation began at day 5 and continued to advanced CHF pathology post-MI. Insufficient scar formation led to rupture or indicated signs of aggravated inflammation within the inflammatory phase reported in many studies or other strains of mice (35). Post-MI, the collagen density was highest in the scar (57 ± 3%), peri-infarct area (30 ± 2%), and to some degree in the remote area (1 ± 0.3%) compared with naïve controls (Fig. 5, A−D). The matured compact fibrotic scar formation is the initiator of the myocardium healing process, but suboptimal scar formation leads to rupture or the excessive matrix accumulation impairs electrical junctions in pathological fibrotic remodeling (35). Extracellular matrix remodeling progresses from AHF to CHF from day 5 onward (AHF) to day 56 (CHF) post-MI, and it indicates irreversible and pathological remodeling.
Fig. 5.
Picrosirius red (PSR)-stained left ventricular (LV) fibrotic remodeling and temporal changes of collagen in remote, peri-infarct, and infarct area in acute heart failure (AHF) and chronic heart failure (CHF) pathology. Representative images of PSR staining depicting fibrosis. A: ×1.25 images of the complete LV. B: ×40 images of the remote zone. C: ×40 images of the peri-infarct zone and quantitative analyses as a bar graph. D: ×40 images of the infarct zone in C57BL/6 mice during AHF and CHF displaying temporal changes in fibrosis during heart failure progression and quantitative analyses as a bar graph (scale bar = 50 µm). n = 7–8 mice/group. *P < 0.05 vs. no-MI control.
Splenic structural remodeling.
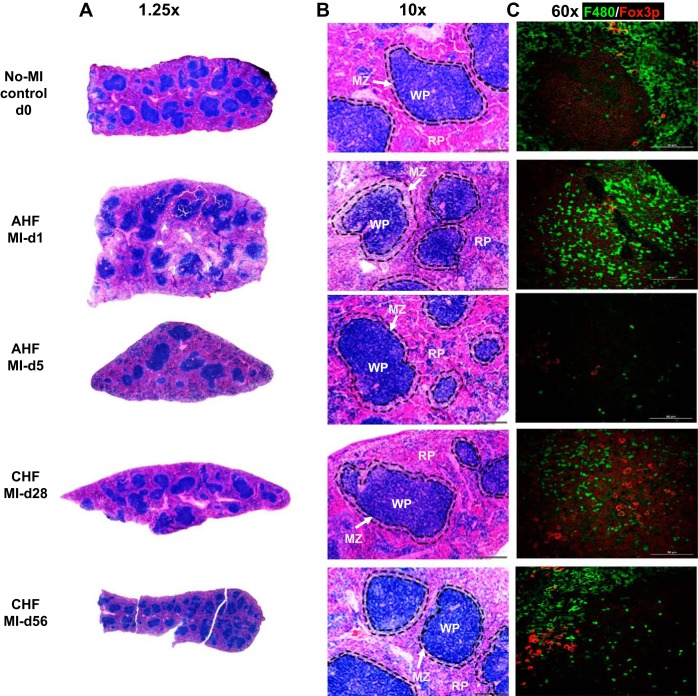
This immune reservoir depletes cells in response to ischemic injury, marked by spleen mass depletion and coordination for the healing response post-MI (Fig. 6 and Table 1). The normal mouse spleen typically has a minor blue tint because of the increased amounts of extramedullary hematopoiesis in the red zone or pulp (RP). In general, the marginal zones (MZ) are variable in size with clear demarcation, and the white pulp (WP; germinal center) is more prominent. During the acute phase (post-MI day 1 to day 5), the spleen showed a decrease in MZ area, losing demarcation with the WP and RP area with shrinkage of the WP area. Furthermore, during CHF pathology MI day 28 and MI day 56, the germinal center (WP) started regaining the original shape as the non-MI naïve spleen (Fig. 6, A and B). Immunofluorescence staining of macrophages expressing F4/80 (green in color) and T cell Foxp3 (forkhead box P3, red) (Fig. 6B) displayed depletion in the leukocyte reservoir during AHF. Monocyte/macrophages (F4/80 positive, expressing green) were mobilized during AHF, and, in contrast, during CHF, the spleen repopulated with an increase in F4/80 (green) and Foxp3+ cells, indicating a bimodal response in splenic remodeling in HF pathology (Fig. 6C). Histological analysis of Foxp3+ indicated the splenic structural remodeling of WP and RP with marked changes in the MZ (Fig. 6C).
Fig. 6.
Sequential changes in splenic remodeling in acute heart failure (AHF) and chronic heart failure (CHF) after myocardial infarction (MI), particularly in the germinal center, marginal zones (MZs), and red pulp (RP) and white pulp (WP). A and B: hematoxylin and eosin-stained images of the spleen from C57BL/6 mice during AHF and CHF displaying temporal changes in the WP area, RP area, and MZ. C: immunofluorescence images representing kinetics of F4/80+ macrophages (green) and Foxp3+ (red) in the spleen in AHF and CHF. Scale bar = 50 µm. Magnification: ×20. n = 7–8 mice/group for hematoxylin and eosin staining and n = 2 for immunofluorescence.
The kidney inflames during AHF and shows structural remodeling during CHF pathology.
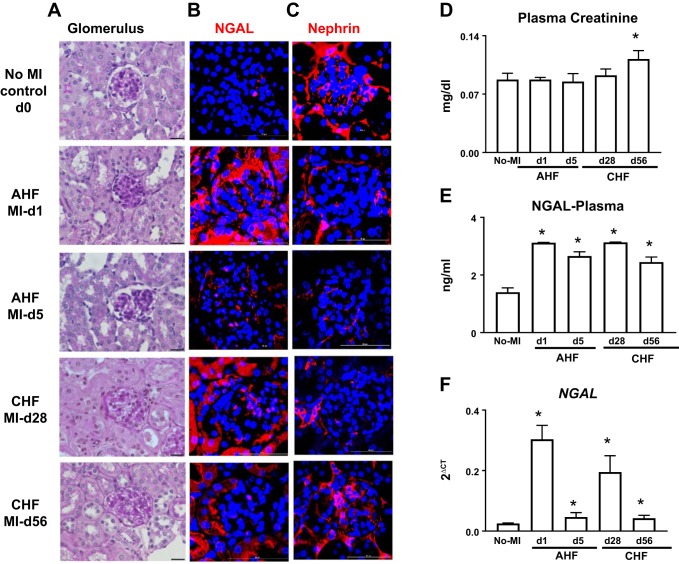
Heart and kidney dysfunction are frequently interdependent, since a dysfunction of one organ often feeds the defective trigger to another organ, with more pronounced damage in both. Evaluation of gross kidney morphology displayed immediate shrinking of the basement membrane at day 1 post-MI to day 5 (AHF), with the expansion of the mesangial matrix distorting glomeruli structure. From post-MI day 28 to day 56 (CHF), the structure of the glomerulus worsened, leaving no space between the basement membrane and mesangial matrix, indicating renal inflammation (Fig. 7A). Post-MI renal inflammation was confirmed by early upregulation of NGAL. Immunofluorescence analysis of NGAL expression (red in color) showed that there was an overall and immediate increase in NGAL expression at day 1 post-MI. In the transition from AHF to CHF (day 5 post-MI), there was a decrease in NGAL gene expression, but the glomerulus consistently displayed expression of NGAL during CHF (day 28 post-MI) indicating a bimodal response (Fig. 7B). Similar changes were detected in mRNA levels of NGAL and plasma (Fig. 7, E and F). Furthermore, the degree of podocyte injury was evaluated during AHF and CHF by immunofluorescence using nephrin. In non-MI naïve control mice, localization of nephrin (red) was observed along the glomerular capillary wall in a continuous pattern, suggesting the localization in podocytes. In MI mice, at day 1 (AHF), the intensity of nephrin diminished, and a further discontinuous pattern was noted along the glomerular capillary at day 5. During CHF, at day 28 the nephrin was diminished but regained expression at day 56 post-MI (Fig. 7C). However, no changes were observed in plasma creatinine levels during AHF (day 1 to day 5 post-MI), but during the chronic phase at day 56, plasma creatinine levels were significantly elevated compared with naïve controls. (Fig. 7D). Thus, MI-induced cardiac damage triggers bimodal kidney-inflamed remodeling in AHF and long-term structural remodeling in AHF and CHF pathology.
Fig. 7.
Periodic acid-Schiff (PAS)-stained renal structural remodeling in acute heart failure (AHF) and chronic heart failure (CHF) pathology with the marked bimodal response of inflammatory markers in the kidney. A: PAS staining indicating granulomatous kidney inflammation in progressive ACF and CHF. B: immunofluorescence images showing immediate increase in neutrophil gelatinase-associated lipocalin (NGAL) expression (red) and nuclei (blue) at day 1 after myocardial infarction (MI) (AHF) followed by the bimodal response at day 28 post-MI (CHF) in the kidney glomerulus and tubular area. C: decrease in nephrin expression (red) in the glomerulus diffused by cardiorenal inflammation during AHF and CHF. Nuclei are stained blue. D: plasma creatinine level in AHF and CHF. E: plasma NGAL levels in AHF and CHF. F: mRNA levels of NGAL in AHF and CHF. Values are means ± SE; n = 6–8 mice/group. Magnification is 3.84 zoom of ×40 for images. Scale bar = 50 µm. n = 7–8 mice/group for PAS staining and n = 2 for immunofluorescence. *P < 0.05 vs. no-MI control.
DISCUSSION
In the present study, we have comprehensively characterized AHF and CHF pathology. In the clinical setting, HF is secondary to MI, which is classified into four stages based on the severity, with class I as the beginning of dysfunction with increased blood pressure and class IV as the end-stage advanced HF with marked discomfort (33, 38). In the preclinical setting, the detailed mechanistic study of molecular and cellular aspects of HF research has expanded in leaps and bounds to develop novel strategies to meet the recent growing population of HF patients. However, there is no consensus for the selection of specific models (ischemia vs. reperfusion) to study structural and functional aspects of AHF and CHF. In a majority of cases, in vitro or in vivo molecular or cellular recovery does not equate to functional, structural, and survival benefits. Therefore, to adopt a comprehensive and integrative approach, we have provided a functional and structural compendium of cardiosplenic and cardiorenal networks in AHF and advanced CHF using the permanent coronary ligation model. Key findings are as follows: 1) LV remodeling is unidirectional, irreversible, and develops nonpreventable chronic pathology with marked signs of wall thinning in AHF and advancing to CHF; 2) transmural fibrotic scarring replaces the infarcted ischemic myocyte area from day 5 onward, with marked LV dilatation and dysfunction in AHF and CHF; 3) the splenic structure is altered, with early loss of marginal zone (splenic leukocyte) and later expansion of WP; and 4) kidney injury markers show a bimodal inflammatory response, with progressive change in glomerulus structure. Our functional and structural compendium of the cardiosplenic and cardiorenal networks in HF will facilitate integrative approaches and provides a platform to study AHF and advanced CHF in cardiac pathology.
Lack of coronary blood flow to the myocardium alters the LV’s electrophysiological, functional, metabolic, and structural responses and develops ischemia (14). Prolonged duration of ischemia or chronic insufficiency of coronary blood flow induces an MI event and subsequent AHF and CHF, with the marked remodeling of the LV. Despite improved survival outcomes after percutaneous coronary vascularization in the clinical setting, the short-term 1-yr mortality and HF rate has increased significantly (25, 36). The translation of cardiac repair in the clinical setting has been both challenging and disappointing because observations in the preclinical setting of molecular or cellular reverse remodeling or the reduction of the infarct area do not necessarily equate with the recovery of myocardial structure and function (12). In most cases, studies are classified into the following two categories: 1) molecular and functional cardiac repair in the ischemic injury setting and 2) molecular and cellular mechanisms of cardiac pathology. In the clinical setting, in contrast, the HF cardiac pathology is heterogeneous, and it alters the cardiosplenic and cardiorenal networks; therefore, a comprehensive and integrative approach is necessary.
William Harvey, the Father of Cardiology, was the first to describe the heart as the main pump of the body to drive blood circulation in the classic monograph “De Motu Cordis” (4). Now, it has been established that the LV wall system is operative in a unique spherical manner that coordinates circumferential, longitudinal, and radial movements of the heart to pump the blood with a definite electrophysiological synchrony (31, 39). Deformation of the LV wall by either hypertrophy (dilated or hypertrophy cardiomyopathy) or wall thinning creates a compensatory mechanism for noncardiomyocyte slippage and fibrogenesis. It remains unclear whether compact fibrosis in the infarct area and patchy scar in the peri-infarct area are pathological or fibroblast-induced compensatory slippage mechanisms of optimal myocardium and fibrotic healing (29). In the presented LV structural and functional compendium, the indepth short- and long-axis temporal analysis clearly differentiates AHF and CHF pathology. The MI-induced cardiosplenic and cardiorenal network compendium will now enable basic scientists and cardiologists to use an ideal model of AHF and comprehensive alteration in the transition of AHF to advanced CHF. The LV unidirectional simplicity, versus the complexity of the integrative network, is also an indicator of heterogeneous pathophysiology. In murine AHF and CHF pathology, the functional and structural similarities are matched to advanced CHF pathology, although CHF mice appear to lack the discomfort felt by HF patients (unpublished observations) (5). Of note, lung function, or the cardiopulmonary axis, has not been evaluated in the present compendium, and that may be an active area of future research.
A second essential aspect of HF pathology is that the cardiosplenic axis needs to be acknowledged as part of the integrative approach. The spleen, as a lymphoid organ, uniquely operates the innate and adaptive immune responses that aid phagocytosis and efferocytosis post-MI (23). Removal of the spleen aggravates LV rupture events, which indicates an essential role for healing, but splenectomy in chronic HF pathology helps to improve LV function and limit inflammation (15, 32). In the present structural compendium, we noticed that there is shrinkage in the germinal center (WP) area during the acute response and that the spleen displays a bimodal response by expanding the WP during CHF. Additional risk factors, such as an obesogenic environment or aging, alter the splenic-derived milieu in the myocardium healing process and are responsible for nonresolving mechanisms in AHF pathology (10). In mice, the expansion of lymphocytes (forkhead transcription factor-T cells) indicates an obvious adaptive response in CHF pathology, but, in humans, the ratio of neutrophils to lymphocytes is increased, indicating differential leukocyte populations in AHF and CHF pathology in mice and humans (19, 24). Quantitative studies using flow cytometry established that, like innate immune cells, Foxp3+ cells coordinate monocyte/macrophage trafficking, phenotypes, and differentiation to facilitate collagen matrix formation in MI-induced and diphtheria toxin receptor models, thereby fostering the healing response during CHF. Adoptive transfer studies have indicated that splenic cells retain memory to induce cardiac injury and pathological remodeling (2, 15, 37). Therefore, the consideration of these differences is critical for the development of novel immunosuppressive or immunogenic targets in AHF and CHF pathology.
A third essential feature of MI-induced complexity is a cardiorenal progression in advanced CHF pathology (7, 22). The kidney glomerulus is the functional unit responsible for ultrafiltration, and it is composed of the capillary endothelium, glomerular basement membrane, and visceral epithelium (30). Traditionally, ischemic myocardial injury and renal dysfunction often coexist in both AHF and CHF pathology, but the degree of failure imposed by one organ that feeds forward the failure of the other organ is unclear (7). Here, we presented progressive temporal changes of glomerulus ultrastructure, particularly in CHF pathology, from day 5 onward that indicates MI-induced cardiorenal structural dysfunction and a bimodal inflammatory response. Although here we focused more on MI-induced cardiorenal pathology, renocardiac pathology is common in the clinical setting because of risk factors such as obesity, hypertension, diabetes, and dyslipidemia or aging. Therefore, consideration of an integrative or comprehensive approach will aid the development of novel treatment strategies (18). Of note, for cardiac pathology or repair with relevance to CHF pathology, the duration of ischemia is the primary variable that determines the extent of myocardium injury. Study of temporal functional, cellular, and histological comparison of 20- versus 40-min ischemia showed that a short duration of ischemia is insufficient to induce fibrosis and advanced CHF after ischemia-reperfusion (9). Therefore, sham-operated control mice were not included in the present comprehensive analyses, since the sham and naïve control mice showed 100% survival. Moreover, sham surgery does not show signs of ischemia that define the naïve control environment (16). Time-dependent myocardial death from the subendocardial region to the less ischemic subepicardial requires 3–6 h of ischemia. Reperfusion after 6-h occlusion or ischemia develops nonsalvageable ischemic myocardium injury in dogs (27). Our findings do not essentially nullify the utility of ischemia- and reperfusion-mediated collateral myocardium injury. Studies in rodents and dogs have shown that 60–90 min of prolonged coronary occlusion results in unidirectional, irreversible, and inflammatory wound healing responses (8). By analogy, the transient (10−30 min) ischemia- and reperfusion-induced injury serves as a “pinching” to the test rodents/primates while permanent coronary ligation (LAD) serves as a sledgehammer “punching” to myocardium that determines the progression to AHF and CHF. To study chronic cardiac pathology or cardiac repair, permanent coronary ligation serves as an optimal model that eliminates the brief ischemia-mediated endogenous reparative mechanisms.
Limitations.
The present AHF and CHF temporal experiments were performed in C57BL/6 male mice at 2–4 mo old. Female C57BL/6 mice or other strains of male and female mice or superimposition with risk factors like obesity, hypertension, diabetes, or aging will have different temporal profiles of AHF and CHF pathology post-MI.
Perspectives and significance.
The modifiable or preventable risk factors, such as environmental exposure (cigarettes or alcohol), comedications (anti-inflammatory or cancer treatment drugs), under- or overnutrition (obesity), sleep and wake cycle (circadian rhythm), and action and inaction (exercise vs. sedentary lifestyle) are prime factors among the many confounders, including aging, that aggravate AHF and CHF pathology. Superimposition of one or more risk factors likely aggravates the diversified cellular pathway and overall structural and functional outcome in AHF and CHF pathology. Therefore, the evolution of the HF specialty from the current focus of thrombocardiologist to nephrocardiologist or pulmnocardiologist or diabetocardiologist or splenocardiologist or gerontocardiologist will ideally serve heterogeneous advanced HF patients.
In summary, the present report provides a LV functional, structural, and histological compendium of the cardiosplenic and cardiorenal networks to study cardiac repair or cardiac pathology that will help improve experimental strategies.
GRANTS
This work was supported by National Institutes of Health Grants AT-006704 and HL-132989 (to G. V. Halade) and by American Heart Association Postdoctoral Fellowship POST31000008 (to V. Kain).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
G.V.H. conceived and designed research; G.V.H., V.K., and K.A.I. performed experiments; G.V.H., V.K., and K.A.I. analyzed data; G.V.H. interpreted results of experiments; G.V.H., V.K., and K.A.I. prepared figures; G.V.H., V.K., and K.A.I. drafted manuscript; G.V.H. edited and revised manuscript; G.V.H. approved final version of manuscript.
REFERENCES
- 1.Ambrosy AP, Fonarow GC, Butler J, Chioncel O, Greene SJ, Vaduganathan M, Nodari S, Lam CSP, Sato N, Shah AN, Gheorghiade M. The global health and economic burden of hospitalizations for heart failure: lessons learned from hospitalized heart failure registries. J Am Coll Cardiol 63: 1123–1133, 2014. doi: 10.1016/j.jacc.2013.11.053. [DOI] [PubMed] [Google Scholar]
- 2.Bansal SS, Ismahil MA, Goel M, Patel B, Hamid T, Rokosh G, Prabhu SD. Activated T lymphocytes are essential drivers of pathological remodeling in ischemic heart failure. Circ Heart Fail 10: e003688, 2017. doi: 10.1161/CIRCHEARTFAILURE.116.003688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bauer M, Cheng S, Jain M, Ngoy S, Theodoropoulos C, Trujillo A, Lin FC, Liao R. Echocardiographic speckle-tracking based strain imaging for rapid cardiovascular phenotyping in mice. Circ Res 108: 908–916, 2011. doi: 10.1161/CIRCRESAHA.110.239574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Braunwald E. Cardiomyopathies: an overview. Circ Res 121: 711–721, 2017. doi: 10.1161/CIRCRESAHA.117.311812. [DOI] [PubMed] [Google Scholar]
- 5.Chauhan C. living with heart failure with preserved ejection fraction. JACC Heart Fail 5: 236–237, 2017. doi: 10.1016/j.jchf.2016.12.013. [DOI] [PubMed] [Google Scholar]
- 6.Chen J, Zhang W, Liu Z, Zhu T, Shen W, Ran J, Tang Q, Gong X, Backman LJ, Chen X, Chen X, Wen F, Ouyang H. Characterization and comparison of post-natal rat Achilles tendon-derived stem cells at different development stages. Sci Rep 6: 22946, 2016. doi: 10.1038/srep22946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Damman K, Testani JM. The kidney in heart failure: an update. Eur Heart J 36: 1437–1444, 2015. doi: 10.1093/eurheartj/ehv010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dewald O, Ren G, Duerr GD, Zoerlein M, Klemm C, Gersch C, Tincey S, Michael LH, Entman ML, Frangogiannis NG. Of mice and dogs: species-specific differences in the inflammatory response following myocardial infarction. Am J Pathol 164: 665–677, 2004. doi: 10.1016/S0002-9440(10)63154-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fernández-Jiménez R, Galán-Arriola C, Sánchez-González J, Agüero J, López-Martín GJ, Gomez-Talavera S, Garcia-Prieto J, Benn A, Molina-Iracheta A, Barreiro-Pérez M, Martin-García A, García-Lunar I, Pizarro G, Sanz J, Sánchez PL, Fuster V, Ibanez B. Effect of ischemia duration and protective interventions on the temporal dynamics of tissue composition after myocardial infarction. Circ Res 121: 439–450, 2017. doi: 10.1161/CIRCRESAHA.117.310901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Halade GV, Kain V, Black LM, Prabhu SD, Ingle KA. Aging dysregulates D- and E-series resolvins to modulate cardiosplenic and cardiorenal network following myocardial infarction. Aging (Albany NY) 8: 2611–2634, 2016. doi: 10.18632/aging.101077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Halade GV, Ma Y, Ramirez TA, Zhang J, Dai Q, Hensler JG, Lopez EF, Ghasemi O, Jin YF, Lindsey ML. Reduced BDNF attenuates inflammation and angiogenesis to improve survival and cardiac function following myocardial infarction in mice. Am J Physiol Heart Circ Physiol 305: H1830–H1842, 2013. doi: 10.1152/ajpheart.00224.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hausenloy DJ, Garcia-Dorado D, Bøtker HE, Davidson SM, Downey J, Engel FB, Jennings R, Lecour S, Leor J, Madonna R, Ovize M, Perrino C, Prunier F, Schulz R, Sluijter JPG, Van Laake LW, Vinten-Johansen J, Yellon DM, Ytrehus K, Heusch G, Ferdinandy P. Novel targets and future strategies for acute cardioprotection: position paper of the European Society of Cardiology Working Group on Cellular Biology of the Heart. Cardiovasc Res 113: 564–585, 2017. doi: 10.1093/cvr/cvx049. [DOI] [PubMed] [Google Scholar]
- 13.Heusch G. critical issues for the translation of cardioprotection. Circ Res 120: 1477–1486, 2017. doi: 10.1161/CIRCRESAHA.117.310820. [DOI] [PubMed] [Google Scholar]
- 14.Heusch G. myocardial ischemia: lack of coronary blood flow or myocardial oxygen supply/demand imbalance? Circ Res 119: 194–196, 2016. doi: 10.1161/CIRCRESAHA.116.308925. [DOI] [PubMed] [Google Scholar]
- 15.Ismahil MA, Hamid T, Bansal SS, Patel B, Kingery JR, Prabhu SD. Remodeling of the mononuclear phagocyte network underlies chronic inflammation and disease progression in heart failure: critical importance of the cardiosplenic axis. Circ Res 114: 266–282, 2014. doi: 10.1161/CIRCRESAHA.113.301720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Iyer RP, de Castro Brás LE, Cannon PL, Ma Y, DeLeon-Pennell KY, Jung M, Flynn ER, Henry JB, Bratton DR, White JA, Fulton LK, Grady AW, Lindsey ML. Defining the sham environment for post-myocardial infarction studies in mice. Am J Physiol Heart Circ Physiol 311: H822–H836, 2016. doi: 10.1152/ajpheart.00067.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kain V, Ingle KA, Colas RA, Dalli J, Prabhu SD, Serhan CN, Joshi M, Halade GV. Resolvin D1 activates the inflammation resolving response at splenic and ventricular site following myocardial infarction leading to improved ventricular function. J Mol Cell Cardiol 84: 24–35, 2015. doi: 10.1016/j.yjmcc.2015.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kingma JG Jr., Simard D, and Rouleau JR. Renocardiac syndromes: physiopathology and treatment stratagems (Abstract). Can J Kidney Health Dis 2: 41, 2015. doi: 10.1186/s40697-015-0075-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kyne L, Hausdorff JM, Knight E, Dukas L, Azhar G, Wei JY. Neutrophilia and congestive heart failure after acute myocardial infarction. Am Heart J 139: 94–100, 2000. doi: 10.1016/S0002-8703(00)90314-4. [DOI] [PubMed] [Google Scholar]
- 20.Lopez EF, Kabarowski JH, Ingle KA, Kain V, Barnes S, Crossman DK, Lindsey ML, Halade GV. Obesity superimposed on aging magnifies inflammation and delays the resolving response after myocardial infarction. Am J Physiol Heart Circ Physiol 308: H269–H280, 2015. doi: 10.1152/ajpheart.00604.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ma Y, Halade GV, Zhang J, Ramirez TA, Levin D, Voorhees A, Jin YF, Han HC, Manicone AM, Lindsey ML. Matrix metalloproteinase-28 deletion exacerbates cardiac dysfunction and rupture after myocardial infarction in mice by inhibiting M2 macrophage activation. Circ Res 112: 675–688, 2013. doi: 10.1161/CIRCRESAHA.111.300502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maisel AS, Katz N, Hillege HL, Shaw A, Zanco P, Bellomo R, Anand I, Anker SD, Aspromonte N, Bagshaw SM, Berl T, Bobek I, Cruz DN, Daliento L, Davenport A, Haapio M, House AA, Mankad S, McCullough P, Mebazaa A, Palazzuoli A, Ponikowski P, Ronco F, Sheinfeld G, Soni S, Vescovo G, Zamperetti N, Ronco C; Acute Dialysis Quality Initiative consensus group . Biomarkers in kidney and heart disease. Nephrol Dial Transplant 26: 62–74, 2011. doi: 10.1093/ndt/gfq647. [DOI] [PubMed] [Google Scholar]
- 23.Mebius RE, Kraal G. Structure and function of the spleen. Nat Rev Immunol 5: 606–616, 2005. doi: 10.1038/nri1669. [DOI] [PubMed] [Google Scholar]
- 24.Mestas J, Hughes CC. Of mice and not men: differences between mouse and human immunology. J Immunol 172: 2731–2738, 2004. doi: 10.4049/jimmunol.172.5.2731. [DOI] [PubMed] [Google Scholar]
- 25.Nabel EG, Braunwald E. A tale of coronary artery disease and myocardial infarction. N Engl J Med 366: 54–63, 2012. doi: 10.1056/NEJMra1112570. [DOI] [PubMed] [Google Scholar]
- 26.Ponikowski P, Anker SD, AlHabib KF, Cowie MR, Force TL, Hu S, Jaarsma T, Krum H, Rastogi V, Rohde LE, Samal UC, Shimokawa H, Budi Siswanto B, Sliwa K, Filippatos G. Heart failure: preventing disease and death worldwide. ESC Heart Fail 1: 4–25, 2014. doi: 10.1002/ehf2.12005. [DOI] [PubMed] [Google Scholar]
- 27.Reimer KA, Jennings RB, Tatum AH. Pathobiology of acute myocardial ischemia: metabolic, functional and ultrastructural studies. Am J Cardiol 52: 72A–81A, 1983. doi: 10.1016/0002-9149(83)90180-7. [DOI] [PubMed] [Google Scholar]
- 28.Roger VL. Epidemiology of heart failure. Circ Res 113: 646–659, 2013. doi: 10.1161/CIRCRESAHA.113.300268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rog-Zielinska EA, Norris RA, Kohl P, Markwald R. The living scar–cardiac fibroblasts and the injured heart. Trends Mol Med 22: 99–114, 2016. doi: 10.1016/j.molmed.2015.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sekulic M, Pichler Sekulic S. A compendium of urinary biomarkers indicative of glomerular podocytopathy. Patholog Res Int 2013: 782395, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Silverman ME. William Harvey and the discovery of the circulation of blood. Clin Cardiol 8: 244–246, 1985. doi: 10.1002/clc.4960080411. [DOI] [PubMed] [Google Scholar]
- 32.Swirski FK, Nahrendorf M. Leukocyte behavior in atherosclerosis, myocardial infarction, and heart failure. Science 339: 161–166, 2013. doi: 10.1126/science.1230719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thygesen K, Alpert JS, Jaffe AS, Simoons ML, Chaitman BR, White HD, Thygesen K, Alpert JS, White HD, Jaffe AS, Katus HA, Apple FS, Lindahl B, Morrow DA, Chaitman BR, Clemmensen PM, Johanson P, Hod H, Underwood R, Bax JJ, Bonow JJ, Pinto F, Gibbons RJ, Fox KA, Atar D, Newby LK, Galvani M, Hamm CW, Uretsky BF, Steg PG, Wijns W, Bassand JP, Menasche P, Ravkilde J, Ohman EM, Antman EM, Wallentin LC, Armstrong PW, Simoons ML, Januzzi JL, Nieminen MS, Gheorghiade M, Filippatos G, Luepker RV, Fortmann SP, Rosamond WD, Levy D, Wood D, Smith SC, Hu D, Lopez-Sendon JL, Robertson RM, Weaver D, Tendera M, Bove AA, Parkhomenko AN, Vasilieva EJ, Mendis S, Bax JJ, Baumgartner H, Ceconi C, Dean V, Deaton C, Fagard R, Funck-Brentano C, Hasdai D, Hoes A, Kirchhof P, Knuuti J, Kolh P, McDonagh T, Moulin C, Popescu BA, Reiner Z, Sechtem U, Sirnes PA, Tendera M, Torbicki A, Vahanian A, Windecker S, Morais J, Aguiar C, Almahmeed W, Arnar DO, Barili F, Bloch KD, Bolger AF, Botker HE, Bozkurt B, Bugiardini R, Cannon C, de Lemos J, Eberli FR, Escobar E, Hlatky M, James S, Kern KB, Moliterno DJ, Mueller C, Neskovic AN, Pieske BM, Schulman SP, Storey RF, Taubert KA, Vranckx P, Wagner DR; Joint ESC/ACCF/AHA/WHF Task Force for Universal Definition of Myocardial Infarction; Authors/Task Force Members Chairpersons; Biomarker Subcommittee; ECG Subcommittee; Imaging Subcommittee; Classification Subcommittee; Intervention Subcommittee; Trials & Registries Subcommittee; Trials & Registries Subcommittee; Trials & Registries Subcommittee; Trials & Registries Subcommittee; ESC Committee for Practice Guidelines (CPG); Document Reviewers . Third universal definition of myocardial infarction. J Am Coll Cardiol 60: 1581–1598, 2012. doi: 10.1016/j.jacc.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 34.Tian Y, Pan D, Chordia MD, French BA, Kron IL, Yang Z. The spleen contributes importantly to myocardial infarct exacerbation during post-ischemic reperfusion in mice via signaling between cardiac HMGB1 and splenic RAGE. Basic Res Cardiol 111: 62, 2016. doi: 10.1007/s00395-016-0583-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.van den Borne SW, van de Schans VA, Strzelecka AE, Vervoort-Peters HT, Lijnen PM, Cleutjens JP, Smits JF, Daemen MJ, Janssen BJ, Blankesteijn WM. Mouse strain determines the outcome of wound healing after myocardial infarction. Cardiovasc Res 84: 273–282, 2009. doi: 10.1093/cvr/cvp207. [DOI] [PubMed] [Google Scholar]
- 36.Velagaleti RS, Pencina MJ, Murabito JM, Wang TJ, Parikh NI, D’Agostino RB, Levy D, Kannel WB, Vasan RS. Long-term trends in the incidence of heart failure after myocardial infarction. Circulation 118: 2057–2062, 2008. doi: 10.1161/CIRCULATIONAHA.108.784215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Weirather J, Hofmann UD, Beyersdorf N, Ramos GC, Vogel B, Frey A, Ertl G, Kerkau T, Frantz S. Foxp3+ CD4+ T cells improve healing after myocardial infarction by modulating monocyte/macrophage differentiation. Circ Res 115: 55–67, 2014. doi: 10.1161/CIRCRESAHA.115.303895. [DOI] [PubMed] [Google Scholar]
- 38.Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr, Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL, Johnson MR, Kasper EK, Levy WC, Masoudi FA, McBride PE, McMurray JJ, Mitchell JE, Peterson PN, Riegel B, Sam F, Stevenson LW, Tang WH, Tsai EJ, Wilkoff BL; American College of Cardiology Foundation; American Heart Association Task Force on Practice Guidelines . 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 62: e147–e239, 2013. doi: 10.1016/j.jacc.2013.05.019. [DOI] [PubMed] [Google Scholar]
- 39.Zhong L, Ghista DN, Tan RS. Left ventricular wall stress compendium. Comput Methods Biomech Biomed Engin 15: 1015–1041, 2012. doi: 10.1080/10255842.2011.569885. [DOI] [PubMed] [Google Scholar]
- 40.Zhou P, Pu WT. Recounting cardiac cellular composition. Circ Res 118: 368–370, 2016. doi: 10.1161/CIRCRESAHA.116.308139. [DOI] [PMC free article] [PubMed] [Google Scholar]