Abstract
The small size of the mouse heart frequently imparts technical challenges when applying conventional in vivo imaging methods for assessing heart function. Here, we describe the use of high-frequency ultrasound imaging in conjunction with a size-tuned blood pool contrast agent for quantitatively assessing myocardial perfusion in living mice. A perflurocarbon microbubble formulation exhibiting a narrow size distribution was developed, and echogenicity was assessed at 18 MHz in vitro. Adult mice were subjected to permanent ligation of the left anterior descending artery. Ultrasound imaging was performed on day 7, and a cohort of intact mice was used as a control. Parasternal long-axis cine clips were acquired at 18 MHz before and after contrast administration. Reduced ejection fraction and increased end-systolic volume were observed in infarcted compared with control mice. In control animals, washin of the contrast agent was visible in all myocardial segments. Reduced contrast enhancement was observed in apical-posterolateral regions of all infarcted mice. A novel method for reslicing of the imaging data through the time domain provided a two-dimensional presentation of regional contrast agent washin, enabling convenient identification of locations exhibiting altered perfusion. Myocardial segments exhibiting diminished contractility were observed to have correspondingly low relative myocardial perfusion. The contrast agent formulation and methods demonstrated here provide the basis for simplifying routine in vivo estimation of infarct size in mice and may be particularly useful in longitudinal evaluation of revascularization interventions and assessment of peri-infarct ischemia.
NEW & NOTEWORTHY Murine myocardial contrast echocardiography frequently suffers from poor sensitivity to contrast. Here, we formulated a novel size-tuned microbubble contrast agent and validated it for use with ultra-high-frequency ultrasound. A novel data method for evaluating myocardial perfusion based on reslicing the imaging data through the time domain is presented.
Keywords: high-frequency ultrasound, microbubble, myocardial contrast echocardiography, myocardial infarction
INTRODUCTION
Noninvasive imaging tools developed specifically for use in the nonclinical research setting have become widely available over the last 15 yr. Such instruments boast higher resolution than their clinical counterparts and therefore enable robust imaging in rodents and other small animals. Contrast media are available for all imaging modalities, and many clinical contrast agents can be used preclinically in rodents at scaled dosage. The use of contrast agents offers tremendous benefit in small animal imaging, due in part to the ease with which investigational imaging agents may be deployed in a research setting. Microbubbles are the dominant contrast agent for ultrasound imaging and are unique in that they are confined to the vasculature by their size (20); therefore, they are attractive for imaging vascular perfusion.
Microbubble-based ultrasound imaging techniques [generally referred to as myocardial contrast echocardiography (MCE)] have been developed for imaging myocardial perfusion in human subjects, although their reverse translation to mice has been problematic. This is in part a function of the very high imaging frequencies (20–50 MHz) used to image mice, which imparts a reduced sensitivity to microbubble contrast agents. Microbubble echogenicity is inversely related to diameter, and microbubble formulations developed for use with low-frequency (2–5 MHz) clinical scanners generally show poor performance when used at high frequency. Additionally, penetration is reduced at high frequency, and shadowing from microbubbles within the left ventricular (LV) chamber frequently prevents assessment of the posterior myocardium during perfusion experiments. This problem is exacerbated in mice exhibiting an enlarged LV dimension, as is found in many models of cardiac dysfunction.
A second difficulty for MCE in a research setting relates to workflow. MCE is susceptible to motion artifacts caused by cardiac and respiratory motion; these problems are amplified in the mouse because of the small spatial dimension. Physiological gating can partially alleviate this, although many studies require some degree of painstaking frame selection and alignment. Although myocardial perfusion imaging has been reported by various groups (10, 18, 28), its widespread adoption has been limited.
In the current study, we present the results of a robust MCE technique for routine use in mice. We used a commercially available small animal ultrasound scanner operating at high imaging frequency and a novel microbubble formulation that exhibits a uniform size distribution enriched in small-diameter particles. The high sensitivity achievable with this microbubble, coupled with the exceptional spatial resolution of high-frequency ultrasound, greatly facilitates use of MCE in the mouse.
METHODS
Ultrasound contrast agents.
A novel microbubble formulation developed for high-frequency imaging was used in these experiments. This agent (TSN-01-011, commercialized for life science use as Targestar P-HF) is a decafluorobutane microbubble encapsulated with a lipid shell. This product was formulated to exhibit a tightly controlled size distribution with very few microbubbles of diameter > 3 μm. A polydisperse microbubble product developed for low-frequency contrast imaging (Targestar-P; see Refs. 6 and 19) was used for in vitro flow phantom experiments. Five commercial lots of TSN-01-011 and one lot of Targestar-P, all prepared at Targeson, were used in this study.
In vitro echogenicity.
In vitro experiments were performed in an acoustic phantom to investigate the suitability of TSN-01-011 for high-frequency imaging. A wall-less agar-gelatin ultrasound phantom was constructed as previously described (2). Briefly, gelatin [6% (wt/wt)] and agar [1.5% (wt/wt)] were sequentially added to warm (90°C) deionized water under agitation, boiled for 5 min, and then cooled for 1 h at room temperature. The mixture was then poured in a polypropylene mold through which a 0.5-mm-diameter length of polyethylene tubing had been placed. The phantom was incubated overnight at 4°C. After solidification, the polyethylene tubing was carefully withdrawn, creating a wall-less flow channel. Luer connectors were inserted in the ends of the channel and sealed with silicon grease. The channel was flushed with degassified saline and incubated for 3 h with casein to prevent nonspecific microbubble adhesion.
The microbubbles were first diluted to a number concentration of 8.6 × 108 particles/ml normal saline. Serial dilutions in saline were then performed, and microbubbles perfused through the flow phantom at 3 ml/min. Imaging was performed on a high-frequency ultrasound scanner marketed for use in small animals (Vevo 2100, Visual Sonics/FUJI). The transducer (MS250S) was clamped above the flow phantom, and the channel was imaged at 18 MHz (power: 10%, mechanical index: 0.1, and dynamic range: 40 dB) in nonlinear contrast imaging mode. Eight 10-fold serial dilutions were prepared and evaluated sequentially. Each dilution was measured in three separate phantoms. The mean contrast enhancement within the flow channel and within an equally sized reference region were measured in 10 representative imaging frames, and the average contrast-to-tissue ratio (CTR) was computed. All quantitative measurements were performed in linearized acoustic units, using densitometry software provided by the scanner manufacturer.
Animal models.
All animal care and experimental procedures were approved by the Institutional Animal Care and Use Committee of the University of California (San Diego, CA). Fourteen healthy male C57/Bl6 mice were used in this study. Seven mice served as intact controls. The remaining mice were subjected to permanent ligation of the left anterior descending (LAD) coronary artery as previously described (16). Briefly, mice were anesthetized with isoflurane, intubated, and ventilated with 1.5–2.0% isoflurane in oxygen. A left anterior thoracotomy was performed using aseptic surgical techniques. The LAD coronary artery was ligated ~2 mm distal to the left atrial appendage with a 6-0 silk suture. Blanching of the myocardium immediately after ligation was used as an indication of complete occlusion. The incision was then closed, and mice were allowed to recover. Mice were imaged on day 7 postligation.
In vivo ultrasound imaging and MCE.
Before being imaged, mice were anesthetized with 1.5% isoflurane in 100% oxygen. Mice were placed supine on an imaging platform at 37°C and secured with surgical tape. Fur from the sternum to diaphragm was removed using a commercial depilatory cream. Simultaneous ECG was acquired using four small-needle electrodes inserted under the skin of all four appendages. A cannula, consisting of a 0.5-in. 28-gauge needle attached to ~4 in. of PE-10 tubing was used to administer contrast agents. The cannula was primed with sterile saline, and the needle was placed in the retroorbital space and secured to the stage with surgical tape. All imaging was performed on a high-frequency ultrasound scanner (Vevo 2100, VisualSonics/FUJI). A linear array transducer (MS 250S) was used. All MCE scans were performed in nonlinear imaging mode, which is a proprietary contrast detection method that enables nondestructive detection of microbubbles (24). The scanner was set so as to display B-mode and contrast images side by side to facilitate subsequent regional analysis. All images were acquired at a transmission frequency of 18 MHz, 10% power, mechanical index of 0.1, and dynamic range of 40 dB. Images were acquired at the highest frame rate possible for each scan area.
The transducer was mounted to a ball-joint clamp attached to a rail apparatus and placed over the thorax of the mouse. A gel standoff of ~1 cm was used to couple the transducer to the skin surface. The imaging stage to which the mouse was secured was tilted ~30° along the anteroposterior axis, and the transducer was aligned so as to obtain a parasternal long-axis view of the heart. The image area was set such that the entire apex and proximal aorta were visible in the field of view. The acoustic focus was placed at the level of the posterior myocardium. The scanner was then placed in nonlinear contrast mode, and the probe placement was adjusted to eliminate imaging artifacts, as required. The real-time ECG trace was integrated on the bottom of the field of view.
The microbubble contrast agent was administered as a bolus of 10 μl, equivalent to a dose of between 2.5 and 3.5 × 109 particles (6.2–8.9 × 109 μm3 of gas). The acquisition of a cine loop was timed so as to have ~2 s of precontrast (baseline) data and the full washin in the same clip (700 frames total, ~20 s). After being imaged, mice were either recovered or euthanized, and hearts were prepared for histology as described below.
Image analysis.
After acquisition, cine loops of the contrast agent washin were exported from the scanner as AVI files. End-systole frames, identified by LV volume, were manually selected and copied to create a new video clip consisting of only end-systole frames. Clips were trimmed to the first frame at which contrast is visible within the LV, which was set to time (t) = 0. Respiration caused a significant motion artifact in some animals, so frames occurring during inspiration were discarded. The true time scale of the gated sequence was reconstructed based on the on-screen frame number and imaging frame rate.
Contrast quantification was performed in ImageJ or MATLAB. A two-dimensional polygonal region of interest (ROI) encompassing the LV was used for the quantification of contrast intensity in this chamber. A one-dimensional ROI, consisting of a segmented line 1 pixel thick, was used for measuring contrast intensity within the myocardium. This ROI was traced beginning at the anteroseptal wall, progressing through the apex, and finishing at the posterolateral wall. Care was taken to position the ROI at the midpoint of the myocardial wall and to avoid epicardial vessels.
Time-intensity analysis was performed as follows. The contrast intensity within the LV in each frame was computed as the mean pixel intensity within the area encompassing the LV ROI. These data were represented by a 1 × N matrix, where N is the number of frames in the sequence. Image intensity in the myocardium was expressed as the intensity at each pixel along the length of the one-dimensional ROI. These data were represented by an M × N matrix, where M is the number of pixels in the ROI (equivalently, a measurement of length of myocardium in the imaging plane) and N is the number of frames. Reslice images were created by slicing the image volume through time domain using a 1-pixel spacing. Resliced images, of dimension M × N, were saved as TIFF files using the scanner color mapping (black-orange-white) and normalized. Time-intensity curves were created in linearized acoustic units for each segment.
A normalized measurement of contrast intensity was used to compare myocardial perfusion between mice. This parameter, referred to as relative perfusion, was computed by dividing the contrast intensity within the myocardium ROI by that of the LV chamber in four end-systolic frames and then averaging. Frames were taken 8 s after contrast agent administration. The fraction of perfused myocardium (and, equivalently, the fraction of nonperfused myocardium) was computed as the fraction of pixels within the myocardial ROI in which the relative perfusion value was above a predetermined threshold. Segmental analysis was performed by dividing the myocardial ROI into six segments of equal length and averaging the pixels within each segment.
LV volume and wall motion analysis.
Three end-diastolic frames and three end-systolic frames from consecutive beats were used to analyze LV volume using in-house software (3). For each beat, the borders of the LV were traced at end diastole and end systole. The border was then divided into 100 equidistant chords, and the distance between the end-diastole and end-systole border tracings was computed. Using the Sheehan method, end-diastolic and end-systolic volumes were calculated as well as ejection fraction. Normalized chord values were computed, with a value of 0.005 set as a threshold for absence of wall motion. A dyssynergy value was computed for each mouse as the fraction of chords with a value of <0.005. The data were rebinned into six segments for comparison with myocardial perfusion data. Assessment of LV function was performed by an operator blinded to the condition and without access to the myocardial perfusion data.
Histology.
Triphenyltetrazolium chloride (TTC) staining was used to identify viable myocardium (4) ex vivo. Briefly, the heart was excised, arrested by retrograde perfusion through the aorta using a modified high-K+ Tyrode solution with 100 mM NaCl, 30 mM KCl, and 10 mM HEPES-NaOH (pH 7.3), and frozen. Using a frame image of the LV echocardiogram and anatomic landmarks as references, a 1-mm-thick longitudinal section correlating to the long-axis ultrasound image was obtained. The section was incubated in PBS with 1% 2,3,5-TTC at 37°C for 30 min, fixed in 10% neutral buffered formalin for 90 min, and photographed. In this method infarcted myocardium exhibits an absence of TTC staining, whereas viable myocardium has a bright red coloration.
Statistics.
All statistical tests were performed in Prism using a significance level of 0.05. Relative perfusion between control and infarcted mice was assessed by the Mann-Whitney U-test. Differences in segmental wall motion and segmental perfusion between control and infarcted mice were assessed by two-way ANOVA using the Bonferroni correction for multiple comparisons. Microbubble stability data were assessed by one-way ANOVA (Kruskal-Wallis).
RESULTS
In vitro echogenicity.
Both the polydisperse (Targestar-P) and uniformly sized microbubble formulations (TSN-01-011) were detectible by contrast imaging in vitro. CTR increased linearly with volume concentration (μl of encapsulated gas/ml of dilution buffer) over five logs for both microbubble formulations. The slope of the regression was significantly (P < 0.05) greater for TSN-01-011 than for Targestar-P, indicating a greater detection sensitivity for this formulation. Shadowing was observed at the highest concentration tested for both products, although this was more severe for Targestar-P than for TSN-01-011 (Fig. 1B).
Fig. 1.
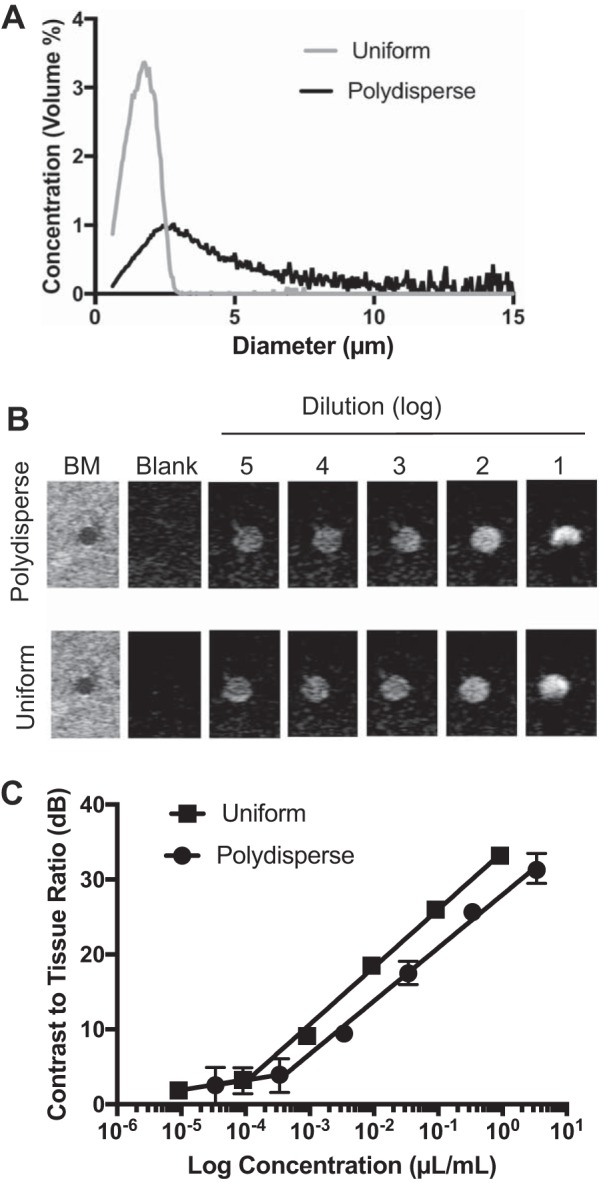
Comparison of in vitro echogenicity of uniform and polydisperse microbubble formulations. A: relative size distributions of standard polydisperse (black line) and uniformly size-tuned (gray line) microbubble products. B: representative ultrasound images for each microbubble product. The first image in each set is a B-mode (BM) of the phantom, and subsequent images are contrast images for serial dilutions. C: contrast-to-tissue ratio as a function of microbubble volume concentration. Lines represent linear fit, and error bars represent range over n = 3 repeated measurements/data point.
Microbubble contrast agent characterization.
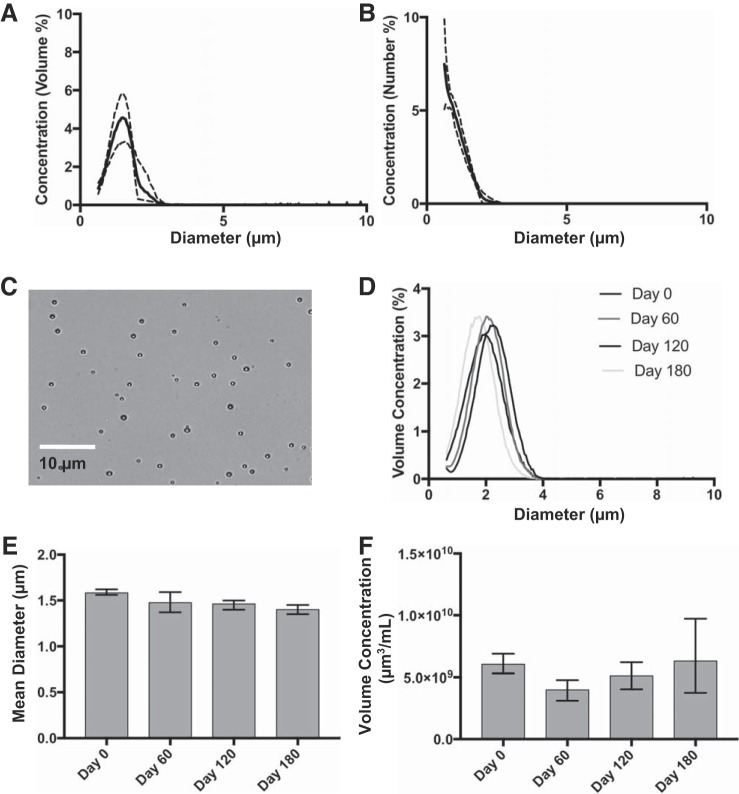
Five lots of TSN-01-011 were subsequently used for in vivo testing. Microbubbles exhibited a mean volume diameter of 1.72 μm (range: 1.56–1.97 μm) and mean number diameter of 1.15 μm (range: 1.08–1.22 μm). The mean size distributions for these product lots in volume and number mode are shown in Fig. 2. Microbubbles were stable on storage at 4°C for up to 6 mo, as demonstrated by the absence of significant change (P > 0.05 by Kruskal-Wallis test) in size distribution, diameter, and concentration (Fig. 2, D–F).
Fig. 2.
Characterization of size-tuned microbubbles used in this study. Size distribution was measured by electrozone sensing for number concentration (A) and volume concentration (B). Traces represent means ± SD of n = 5 microbubble product lots. C: representative transillumination micrograph of microbubbles. Measurements from a single lot over 180 days are shown for overlay of volumetric size distributions (D), mean diameter (E), and microbubble volume concentration (F). Error bars represent the range of n = 4 vials/time point.
Wall motion analysis.
All 14 mice survived the surgical procedure and subsequent ultrasound imaging procedure. Mice that underwent permanent surgical LAD ligation (the infarcted cohort) exhibited significantly increased LV volume that was apparent by B-mode echocardiography. Complete visualization of the parasternal long axis was occasionally challenging for the infarcted cohort because of the size of the heart, and optimization of the probe placement was required for each animal. Dyssynergy calculations were not possible in one control mouse and two infarcted mice because of inadequate visualization. The spatial resolution varied between mice because of the positioning of the transducer, although there was not a statistically significant difference in spatial resolution between the cohorts (Table 1).
Table 1.
Summary of wall motion and perfusion parameters for infarction and control mouse cohorts
| Parameter | Control Mice | Infarcted Mice |
|---|---|---|
| Spatial resolution, pixel/mm | 27.7 ± 2.43 | 31.4 ± 5.56 |
| End-systolic volume, μl | 60.03 ± 38.81 | 111.77 ± 52.13 |
| Ejection fraction, % | 44.4 ± 16.0 | 24.5 ± 5.4 |
| Dyssynergy, % | 2.0 ± 3.0 | 25 ± 11 |
Wall motion analysis revealed a significant reduction in ejection fraction (P = 0.02) and increased dyssynergy (P = 0.009) and end-diastolic volume (P = 0.03) in the infarcted cohort relative to the intact control cohort. No change in end-systolic volume (P = 0.18) was observed (Table 1).
In vivo MCE.
Precontrast images in nonlinear contrast mode showed occasional artifacts at the skin line and epicardium, although baseline intensity within the myocardium was uniformly low in all segments (Fig. 3A). Administration of the contrast agent as a 10-μl bolus through the retroorbital sinus was successful in all mice examined. In control mice, agent was visible within the right heart in <2 s after administration.
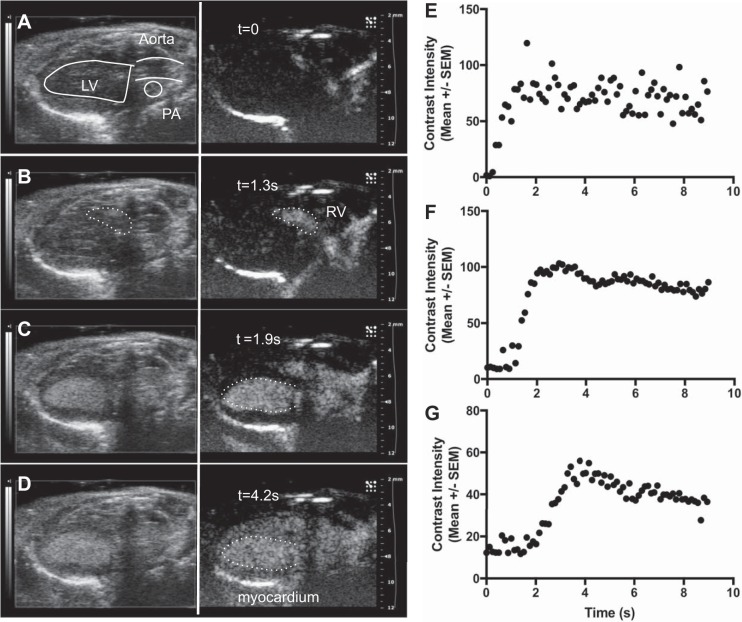
Fig. 3.
Representative time course of contrast activity in the intact (control) murine heart after intravenous bolus administration. Images depict B-mode (left) and corresponding contrast mode (right) at indicated time points as follows: at time (t) = 0 (immediately before contrast injection, A), t = 1.3 s [contrast is visible in the right ventricle (RV) B], t = 2.4 s [full enhancement of the left ventricle (LV), C], and t = 5.2 s (full enhancement of myocardium, D). The white broken lines in C and D show the LV border. Time-intensity curves are plotted for the RV (E), LV (F), and myocardium (G) and show the sequential movement of the contrast bolus through the heart. Error bars represent SE of contrast intensity within the region of interest.
Transit of the contrast agent through the right and left heart after intravenous administration was readily appreciated in the parasternal long-axis view (Fig. 3, B–D). The contrast signal was visible in real time, without the need for offline tissue subtraction or other postprocessing. Both ventricles and the myocardium showed a rapid increase in contrast intensity followed by a period of sustained enhancement, as illustrated by time-intensity curves (Fig. 3, E–G). Contrast signal was visible within the myocardium for up to 2 min after bolus administration and up to 12 min in the LV chamber.
The small size of the mouse heart relative to displacement caused by cardiac and respiratory motion made reproducible placement of two-dimensional ROI difficult. We therefore chose to use an alternative ROI strategy in which a line of 1 pixel width was traced at the center of the myocardium thickness around the contour of the myocardium in long axis (Fig. 4, A and E). This method enabled reslicing the image set through the time domain. This results in a two-dimensional image in which location along the myocardium is represented in one dimension and time is represented in the second dimesion (Fig. 4, D and H). This provides a graphical tool to visualize time-intensity behavior, both contrast washin rate and the magnitude of the contrast signal.
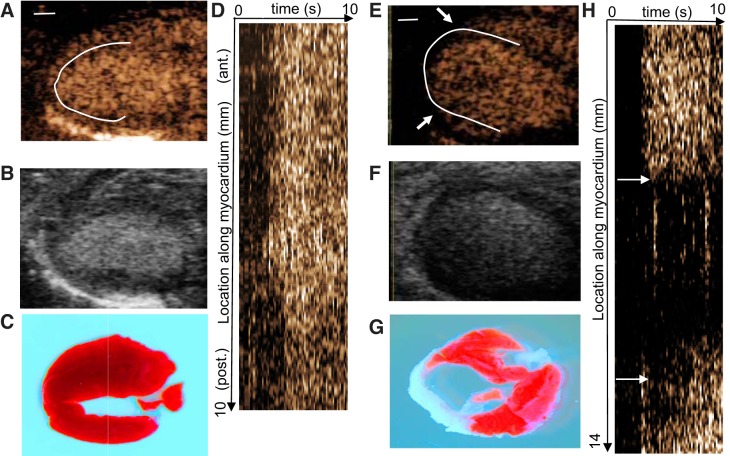
Fig. 4.
Reslice analysis in control and infarcted mice. Representative contrast images at time (t) = 8 s are shown for a control (A) and an infarcted (E) mouse. The white solid line represents the one-dimensional region of interest (ROI) used to create the reslice images. Arrows in E and H denote the extent of the perfusion defect. Corresponding B-mode images and triphenyltetrazolium chloride (TTC) stains are shown in B and F and in C and G, respectively. Red TTC staining represents viable myocardium, whereas white regions are nonviable. Reslice images, depicting contrast intensity along the length of the ROI (vertical dimension) over time (horizontal dimension) are shown in D and H.
In the control mouse example shown in Fig. 4D, relatively uniform enhancement is observed over the entire myocardium, with all segments filling at approximately the same time (~2 s). In the infarcted animal shown in Fig. 4H, the anteroseptal myocardium exhibits uniform washin of contrast. An abrupt loss in contrast signal is noted in the apical region (~5–11 mm). Delayed enhancement is observed in the mid posterolateral myocardium, and homogeneous enhancement is observed at the basal segment of the posterolateral wall. The spatial location of absent and delayed contrast washin corresponds to regions of necrosis by TTC staining (Fig. 4, C and G), as has been found in other studies (5, 7, 33).
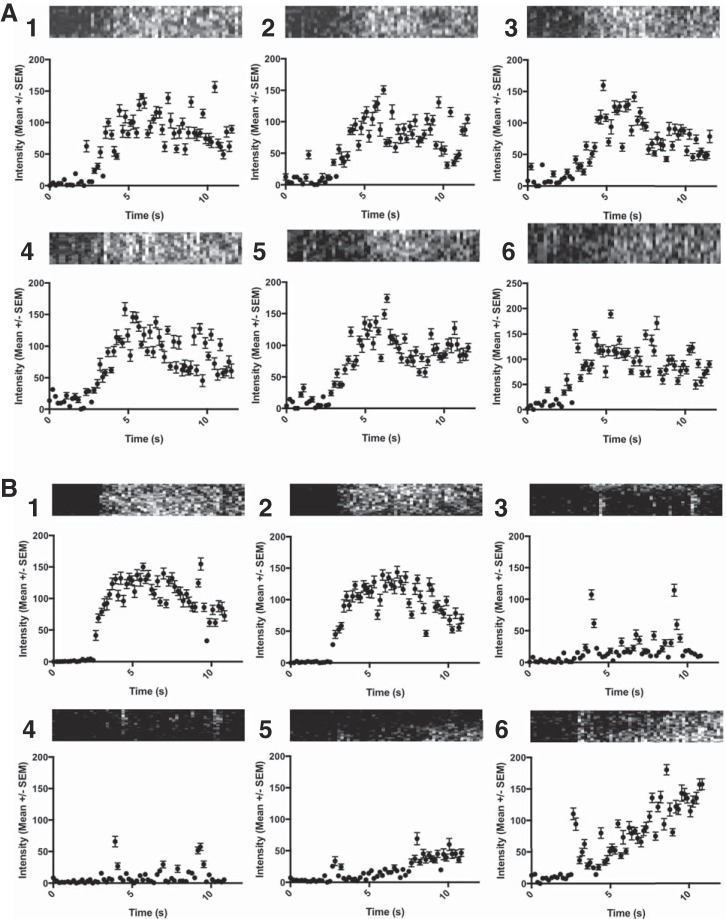
The reslice images provided a graphical interpretation of the time-intensity curve. Figure 5 shows time-intensity curves derived from the reslice images shown in Fig. 4. The myocardium is divided into six segments, and the mean intensity within each segment is plotted as a function of time. Similar time-intensity behavior was observed in all segments in the control mouse (Fig. 4A), with peak intensity reached at ~5 s followed by a slight decrease. Similar behavior was observed in segments 1 and 2 in the infarcted mouse (Fig. 4B). No meaningful time-intensity trend was observed in segments 3 and 4, demonstrating absence of contrast entering these segments. The shape of the time-intensity curves for segments 5 and 6 was distinct from the anteroseptal regions, exhibiting a slower rise and slightly reduced peak intensity. Reslice images from other animals are shown in Supplemental Fig. S1 in the Supplemental Material and show the variability in the location and degree of perfusion defect in infarcted mice (Supplemental Material for this article is available at the American Journal of Physiology-Heart and Circulatory Physiology website).
Fig. 5.
Time-intensity curves derived for the control (A) and infarcted (B) mice examined in Fig. 4. The linear myocardium ROI was divided into 6 segments of equal length, labeled as 1–6. Data are presented as mean intensities ± SE within the ROI. Reslice images representing the portion of myocardium contained in each segment are presented above each curve.
Regional analysis.
We next sought a robust metric for quantifying the extent of nonperfused myocardium, which in this model is expected to correspond to nonviable tissue. To this end, we sought to quantify the amount of contrast material that can perfuse a given segment of myocardium rather than the rate at which it perfused. MCE images were analyzed 8 s after the administration of contrast, at which point the contrast material had sufficient time to enter all regions of perfused myocardium but before significant clearance of the agent. Relative perfusion was computed by normalizing the myocardial intensity by that within the LV, which enabled robust comparison between subjects. Figure 6A shows a histogram of relative perfusion values pooled for the control and infarcted cohorts (n = 7 mice in each cohort). The shape of the histograms is noticeably different, with a significant proportion of values in the infarcted cohort exhibiting low relative perfusion values. A relative perfusion value of 0.07 was chosen as a threshold, with pixels exhibiting a relative perfusion of >0.07 being classified as perfused and those <0.07 classified as nonperfused. The threshold value was selected from the control mouse cohort, for which >99% of pixels exhibited a relative perfusion value of >0.07.
Fig. 6.
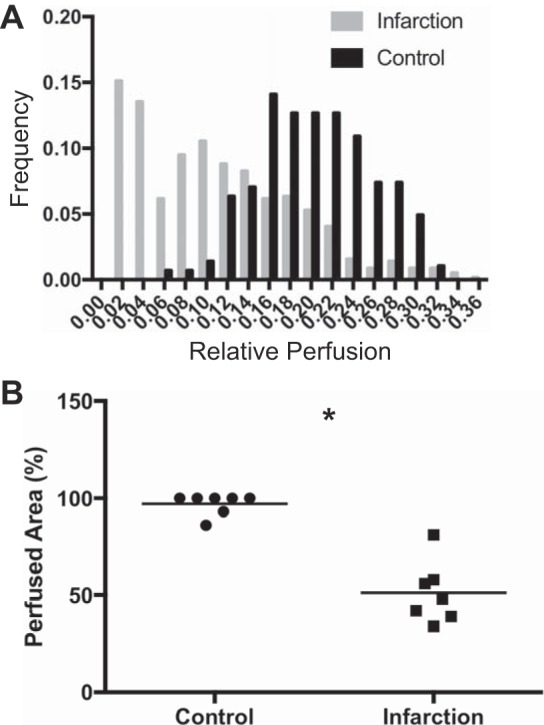
Comparison of relative myocardial perfusion. A: pooled histogram for control (black bars) and infarcted (gray bars) cohorts. B: scatter plot depicting area fraction of actively perfused myocardium area for each cohort. Bars represent mean of n = 7 animals/cohort. *P < 0.01 by Mann-Whitney.
The perfused area, which is a global measurement of myocardial perfusion defined as the proportion of pixels in each myocardial ROI exhibiting a relative perfusion value >0.07, was computed for each mouse. The mean fractional perfusion for the control cohort was nearly 100% (97 ± 5.0%), as would be expected, since this cohort was used to define full perfusion. The mean fractional perfusion of the infarcted cohort was 53 ± 17%, which was significantly (P > 0.001) less than that of the control cohort (Fig. 6B). The inverse of perfused area, referred to as nonperfused area, was linearly related to dyssynergy (data not shown).
The fractional perfusion and wall motion analyses were subsequently performed on a segmental basis. There were no significant differences in fractional chord shortening or relative perfusion between any segments in the control mouse cohort. In the infarcted cohort, fractional shortening in segments 3 and 4 was significantly lower (P < 0.05) than that in the same segments of the control cohort (Fig. 7A). The relative perfusion in the infarcted cohort was significantly lower (P < 0.05) in segments 3−5 (Fig. 7B). A scatterplot relating fractional shortening and relative perfusion on a segmental basis revealed two populations: one population containing low relative perfusion (<0.1) and shortening (<0.005) and containing only segments from the infarcted cohort and a second population consisting of segments having a relative perfusion between 0.1 and 0.3 and fractional chord shortening of between 0.01 and 0.04 (Fig. 7C).
Fig. 7.
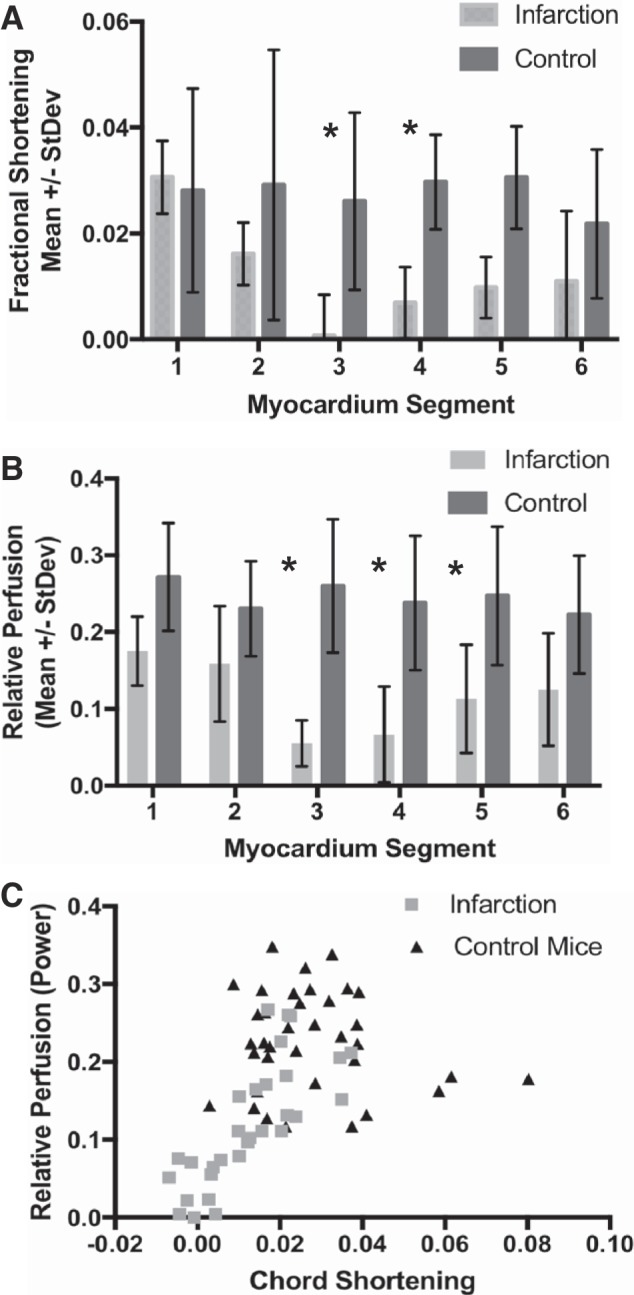
Fractional shortening and perfusion analysis of 6-segment myocardium model. Fractional shortening (A) and relative myocardial perfusion (B) as a function of segment for infarction (gray) and control (black) mouse cohorts. C: scatter plot relating relative perfusion and chord shortening for each segment in control (black triangles) and infarction (gray squares) mice. *P < 0.05 by 2-way ANOVA with Bonferroni correction.
DISCUSSION
In the present study, we explored a new MCE approach for measuring myocardial perfusion in mice, in particular to quantify the spatial extent of myocardial necrosis. Several significant advances were introduced relative to previous studies: we used a high-frequency ultrasound system capable of sensitive nonlinear contrast imaging, we developed a size-tuned perfusion contrast agent designed to operate at high frequencies, and we used a novel ROI placement that enables time-domain reslicing. We demonstrated that contrast could be well visualized in all myocardial segments, including the posterolateral segments deep to the LV. Furthermore, we developed a robust method for representing time-intensity data in graphical form, which facilitates visualization of relative perfusion levels throughout the myocardium. The methods, instruments, and reagents described here may be useful tools that enable this technique to be routinely used in life science research.
Microbubble contrast agents have a long history in echocardiography. All three microbubble drug products currently approved in the United States carry an indication for LV opacification, which can aid in delineation of endocardial borders in some patients. The use of microbubbles for evaluation of myocardial perfusion (34) has been evaluated in various settings, although no clinical approval for this indication has been issued in the United States. The diagnostic accuracy of MCE in identifying perfusion abnormalities secondary to coronary artery disease has been established with reference to 99mTC Sestamibi SPECT or coronary angiography in several large clinical studies (1, 11, 29, 30). MCE has been demonstrated to add incremental diagnostic and prognostic information over wall motion analysis for detection of coronary artery disease on stress exam (11, 23). Finally, MCE has been used to assess microvascular integrity (25, 31) after myocardial infarction (MI) and to predict recovery after reperfusion therapy (12, 22).
The most practical applications for MCE in experimental research diverge somewhat from those used in patients. In the research setting, studies are typically performed on cohorts of identically treated animals, and the ability to detect differences between treatment groups (rather than characterize disease in a single subject) is desired. Important research applications for MCE in small animals include phenotyping animal models (26, 27) and assessing response to experimental therapy (15, 21). The spatial extent of myocardium at risk or infarcted can be used as a powerful and versatile end point in many studies.
In animal models, the spatial distribution of infarction is typically assessed ex vivo using enzymatically cleavable dyes such as tetrazolium chloride. Noninvasive methods that do not require euthanization of the animal are preferable for many study designs, since they enable longitudinal assessment of the subjects. Ultrasound is well suited for imaging the mouse heart because of its high temporal and spatial resolution and the availability of a wide range of blood pool and molecularly targeted contrast agents (14). Heart volume measurements and systolic functional analyses can be performed routinely in small animals and can identify animals with prior MI (13, 17, 35). However, such techniques are occasionally problematic for the assessment of infarction size or location. For example, wall motion may overestimate the extent of infarction (7) resulting from transient effects such as myocardial stunning.
Necrotic myocardium does not have an intact microcirculation, which suggests the utility of intravascular contrast agents (such as microbubbles) for indirectly assessing regional myocardial necrosis. Indeed, numerous studies have documented a strong correlation between infarct area identified by MCE and TTC staining (5, 7, 33) in various animal models of MI. However, MCE is not widely used in mice, due in part to the difficulty that imaging the diminutive mouse heart entails.
The advent of ultrasound scanners capable of using ultra-high frequency (15–40 MHz) has been a boon for small animal imaging (9). However, the sensitivity to contrast at these frequencies is dramatically reduced, requiring high doses of contrast agent. In the mouse heart, acoustic shadowing caused by contrast within the LV cavity can obscure the signal from posterior myocardium. A clever solution to this problem was proposed by Kaufmann et al. (18), who used a large (3.6% of microbubbles of diameter > 5 μm) microbubble formulation designed to be retained by lodging in the myocardial capillaries (but not within the LV). Other investigators (8, 32) have used similarly large (mean diameter: 6–8 μm) microbubbles, which scatter a high amount of ultrasound and thus provide an improved echo signal on B-mode imaging. Neither strategy, however, enables contrast-specific imaging; rather, the echo signal from the microbubble contrast agent is derived by digital subtraction. This limits the sensitivity of the technique, especially in organs with extensive motion such as the heart.
In the present study, we chose to formulate a microbubble suitable for nonlinear imaging at high frequencies. To this end, we created a microbubble exhibiting a smaller mean diameter than conventional microbubble contrast agents (Fig. 1A). Additionally, the size distribution of the microbubble contrast agent also plays a key role in the detection sensitivity of contrast echocardiography. Microbubble formulations used clinically are highly polydisperse, containing agents of diameter between 1 and 10 μm. This can be considered a useful property, since it enables the agent to be useful over a range of imaging frequencies, conditions, scanner models, and contrast detection methods. However, the presence of large (high-diameter) microbubbles can contribute to acoustic shadowing, to which MCE is particularly susceptible in mice. We therefore formulated the microbubble with a very tight size distribution in which essentially no microbubbles of diameter > 3 μm were present. This formulation proved efficacious for imaging at 18 MHz, which was selected to provide a reasonable balance of spatial resolution and contrast sensitivity.
The identification of necrosis, as opposed to a perfusion defect caused by a flow-limiting stenosis, relies on the steady-state phase of contrast enhancement that occurs after washin. Because most of the myocardial blood volume is found within the capillaries, the steady-state contrast enhancement image is essentially a representation of the microvascular blood volume (31). This makes for a relatively simple imaging procedure in that it does not necessarily require derivation of regional time-intensity curves for all animals. We observed a delay in the time required to observe myocardial contrast enhancement in some infarcted mice, presumably secondary to impaired heart function in these animals. Myocardial enhancement was observed in all animals between 2 and 8 s after bolus administration. Our protocol consisted of acquiring four end-systolic frames taken 8 s after bolus administration of the contrast agent. This served to greatly improve throughput, both during imaging and offline analysis.
We used a 1-pixel-thick ROI, placed at the center of the myocardial thickness, to assess myocardial perfusion. This was done in part to enable time domain reslicing but also in response to the thinning observed in the apical myocardium. At the spatial resolution provided at 18 MHz, drawing more substantial ROIs is problematic, often requiring careful repositioning to avoid contamination from the LV chamber. The mouse model used in the present study (7 days after permanent LAD ligation) exhibits transmural necrosis (Fig. 4G), making the position of the ROI within the wall thickness largely irrelevant. However, there is a possibility of sampling artifact in viable regions, and it is therefore not certain whether the subtle variations in contrast intensity observed throughout the myocardium in the control cohort represent actual variations in blood volume or are the result of the limited ROI.
There are several limitations in this study. Our analysis method was evaluated for a single, although widely used, model of MI. Further evaluation, particularly in mouse models involving varying degrees of MI and at different time points, is warranted. The spatial resolution available at 18 MHz did not allow for the assessment of infarct transmurality. Full-thickness infarcts were likely present in the model (7 days after LAD ligation) used here, although our MCE imaging technique may have limited sensitivity in other models of MI. We used the retroorbital route for contrast administration. This minimally invasive method is relatively easy and noninvasive, although it may not provide the precise control of dosing that can be obtained by jugular cannulas (18, 28). Finally, analysis was based on a two-dimensional single field of view rather than the full volume of the heart. Introduction of a high-frequency contrast-enabled volumetric transducer would dramatically increase the power of this technique for small animal work.
GRANTS
This study was supported by National Institutes of Health Grants CA-153618 (J. Rychak), HL-110496 (J. Rychak), and HL-46345-16 (K. Peterson) and by the Jack Tang Memorial Fund.
DISCLOSURES
J. Rychak, A. Luong, and D. Smith disclose employment in Targeson. J. Rychak discloses equity in Targeson. Additional funding for this study was provided by Targeson.
AUTHOR CONTRIBUTIONS
E.A., N.D.D., Y.G., D.S., A.L., M.H., and J.R. performed experiments; E.A., N.D.D., D.S., A.L., and J.R. analyzed data; E.A., K.L.P., and J.R. interpreted results of experiments; E.A. and J.R. prepared figures; E.A. and J.R. drafted manuscript; E.A., K.L.P., and J.R. edited and revised manuscript; E.A., N.D.D., D.S., A.L., K.L.P., and J.R. approved final version of manuscript; J.R. conceived and designed research.
Supplemental Data
REFERENCES
- 1.Abdelmoneim SS, Bernier M, Dhoble A, Moir S, Hagen ME, Ness SA, Pellikka PA, Abdel-Kader SS, Mulvagh SL. Diagnostic accuracy of contrast echocardiography during adenosine stress for detection of abnormal myocardial perfusion: a prospective comparison with technetium-99 m sestamibi single-photon emission computed tomography. Heart Vessels 25: 121–130, 2010. doi: 10.1007/s00380-009-1174-x. [DOI] [PubMed] [Google Scholar]
- 2.Anderson CR, Rychak JJ, Backer M, Backer J, Ley K, Klibanov AL. scVEGF microbubble ultrasound contrast agents: a novel probe for ultrasound molecular imaging of tumor angiogenesis. Invest Radiol 45: 579–585, 2010. doi: 10.1097/RLI.0b013e3181efd581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bale TL, Hoshijima M, Gu Y, Dalton N, Anderson KR, Lee K-F, Rivier J, Chien KR, Vale WW, Peterson KL. The cardiovascular physiologic actions of urocortin II: acute effects in murine heart failure. Proc Natl Acad Sci USA 101: 3697–3702, 2004. doi: 10.1073/pnas.0307324101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bohl S, Medway DJ, Schulz-Menger J, Schneider JE, Neubauer S, Lygate CA. Refined approach for quantification of in vivo ischemia-reperfusion injury in the mouse heart. Am J Physiol Heart Circ Physiol 297: H2054–H2058, 2009. doi: 10.1152/ajpheart.00836.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen X, Cui K, Xiu J, Lin H, Lao Y, Zhou B, Liang F, Zha D, Bin J, Liu Y. Evaluation and simplified measurement of infarct size by myocardial contrast echocardiography in a rat model of myocardial infarction. Int J Cardiovasc Imaging 25: 713–716, 2009. doi: 10.1007/s10554-009-9474-x. [DOI] [PubMed] [Google Scholar]
- 6.Declèves AE, Rychak JJ, Smith DJ, Sharma K. Effects of high-fat diet and losartan on renal cortical blood flow using contrast ultrasound imaging. Am J Physiol Renal Physiol 305: F1343–F1351, 2013. doi: 10.1152/ajprenal.00326.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dourado PM, Tsutsui JM, Mathias W Jr, Andrade JL, da Luz PL, Chagas AC. Evaluation of stunned and infarcted canine myocardium by real time myocardial contrast echocardiography. Braz J Med Biol Res 36: 1501–1509, 2003. doi: 10.1590/S0100-879X2003001100009. [DOI] [PubMed] [Google Scholar]
- 8.Feshitan JA, Chen CC, Kwan JJ, Borden MA. Microbubble size isolation by differential centrifugation. J Colloid Interface Sci 329: 316–324, 2009. doi: 10.1016/j.jcis.2008.09.066. [DOI] [PubMed] [Google Scholar]
- 9.Foster FS, Hossack J, Adamson SL. Micro-ultrasound for preclinical imaging. Interface Focus 1: 576–601, 2011. doi: 10.1098/rsfs.2011.0037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.French BA, Li Y, Klibanov AL, Yang Z, Hossack JA. 3D perfusion mapping in post-infarct mice using myocardial contrast echocardiography. Ultrasound Med Biol 32: 805–815, 2006. doi: 10.1016/j.ultrasmedbio.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 11.Gaibazzi N, Rigo F, Reverberi C. Detection of coronary artery disease by combined assessment of wall motion, myocardial perfusion and coronary flow reserve: a multiparametric contrast stress-echocardiography study. J Am Soc Echocardiogr 23: 1242–1250, 2010. doi: 10.1016/j.echo.2010.09.003. [DOI] [PubMed] [Google Scholar]
- 12.Galiuto L, Garramone B, Scarà A, Rebuzzi AG, Crea F, La Torre G, Funaro S, Madonna M, Fedele F, Agati L; AMICI Investigators . The extent of microvascular damage during myocardial contrast echocardiography is superior to other known indexes of post-infarct reperfusion in predicting left ventricular remodeling: results of the multicenter AMICI study. J Am Coll Cardiol 51: 552–559, 2008. doi: 10.1016/j.jacc.2007.09.051. [DOI] [PubMed] [Google Scholar]
- 13.Gao XM, Dart AM, Dewar E, Jennings G, Du XJ. Serial echocardiographic assessment of left ventricular dimensions and function after myocardial infarction in mice. Cardiovasc Res 45: 330–338, 2000. doi: 10.1016/S0008-6363(99)00274-6. [DOI] [PubMed] [Google Scholar]
- 14.Hyvelin JM, Tardy I, Arbogast C, Costa M, Emmel P, Helbert A, Theraulaz M, Nunn AD, Tranquart F. Use of ultrasound contrast agent microbubbles in preclinical research: recommendations for small animal imaging. Invest Radiol 48: 570–583, 2013. doi: 10.1097/RLI.0b013e318289f854. [DOI] [PubMed] [Google Scholar]
- 15.Inaba Y, Davidson BP, Kim S, Liu YN, Packwood W, Belcik JT, Xie A, Lindner JR. Echocardiographic evaluation of the effects of stem cell therapy on perfusion and function in ischemic cardiomyopathy. J Am Soc Echocardiogr 27: 192–199, 2014. doi: 10.1016/j.echo.2013.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Iwanaga Y, Hoshijima M, Gu Y, Iwatate M, Dieterle T, Ikeda Y, Date MO, Chrast J, Matsuzaki M, Peterson KL, Chien KR, Ross J Jr. Chronic phospholamban inhibition prevents progressive cardiac dysfunction and pathological remodeling after infarction in rats. J Clin Invest 113: 727–736, 2004. doi: 10.1172/JCI18716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kanno S, Lerner DL, Schuessler RB, Betsuyaku T, Yamada KA, Saffitz JE, Kovacs A. Echocardiographic evaluation of ventricular remodeling in a mouse model of myocardial infarction. J Am Soc Echocardiogr 15: 601–609, 2002. doi: 10.1067/mje.2002.117560. [DOI] [PubMed] [Google Scholar]
- 18.Kaufmann BA, Lankford M, Behm CZ, French BA, Klibanov AL, Xu Y, Lindner JR. High-resolution myocardial perfusion imaging in mice with high-frequency echocardiographic detection of a depot contrast agent. J Am Soc Echocardiogr 20: 136–143, 2007. doi: 10.1016/j.echo.2006.08.008. [DOI] [PubMed] [Google Scholar]
- 19.Kumar KN, Sarkar K. Interfacial rheological properties of contrast microbubble Targestar P as a function of ambient pressure. Ultrasound Med Biol 42: 1010–1017, 2016. doi: 10.1016/j.ultrasmedbio.2015.11.017. [DOI] [PubMed] [Google Scholar]
- 20.Lindner JR, Song J, Jayaweera AR, Sklenar J, Kaul S. Microvascular rheology of Definity microbubbles after intra-arterial and intravenous administration. J Am Soc Echocardiogr 15: 396–403, 2002. doi: 10.1067/mje.2002.117290. [DOI] [PubMed] [Google Scholar]
- 21.Ma L, Chuang CC, Weng W, Zhao L, Zheng Y, Zhang J, Zuo L. Paeonol protects rat heart by improving regional blood perfusion during no-reflow. Front Physiol 7: 298, 2016. doi: 10.3389/fphys.2016.00298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Main ML, Magalski A, Morris BA, Coen MM, Skolnick DG, Good TH. Combined assessment of microvascular integrity and contractile reserve improves differentiation of stunning and necrosis after acute anterior wall myocardial infarction. J Am Coll Cardiol 40: 1079–1084, 2002. doi: 10.1016/S0735-1097(02)02124-1. [DOI] [PubMed] [Google Scholar]
- 23.Moir S, Haluska BA, Jenkins C, Fathi R, Marwick TH. Incremental benefit of myocardial contrast to combined dipyridamole-exercise stress echocardiography for the assessment of coronary artery disease. Circulation 110: 1108–1113, 2004. doi: 10.1161/01.CIR.0000139905.47128.9F. [DOI] [PubMed] [Google Scholar]
- 24.Needles A, Arditi M, Rognin NG, Mehi J, Coulthard T, Bilan-Tracey C, Gaud E, Frinking P, Hirson D, Foster FS. Nonlinear contrast imaging with an array-based micro-ultrasound system. Ultrasound Med Biol 36: 2097–2106, 2010. doi: 10.1016/j.ultrasmedbio.2010.08.012. [DOI] [PubMed] [Google Scholar]
- 25.Ragosta M, Powers ER, Samady H, Gimple LW, Sarembock IJ, Beller GA. Relationship between extent of residual myocardial viability and coronary flow reserve in patients with recent myocardial infarction. Am Heart J 141: 456–462, 2001. doi: 10.1067/mhj.2001.113074. [DOI] [PubMed] [Google Scholar]
- 26.Raher MJ, Thibault H, Poh KK, Liu R, Halpern EF, Derumeaux G, Ichinose F, Zapol WM, Bloch KD, Picard MH, Scherrer-Crosbie M. In vivo characterization of murine myocardial perfusion with myocardial contrast echocardiography: validation and application in nitric oxide synthase 3 deficient mice. Circulation 116: 1250–1257, 2007. doi: 10.1161/CIRCULATIONAHA.107.707737. [DOI] [PubMed] [Google Scholar]
- 27.Redfors B, Shao Y, Wikström J, Lyon AR, Oldfors A, Gan LM, Omerovic E. Contrast echocardiography reveals apparently normal coronary perfusion in a rat model of stress-induced (Takotsubo) cardiomyopathy. Eur Heart J Cardiovasc Imaging 15: 152–157, 2014. doi: 10.1093/ehjci/jet079. [DOI] [PubMed] [Google Scholar]
- 28.Scherrer-Crosbie M, Steudel W, Ullrich R, Hunziker PR, Liel-Cohen N, Newell J, Zaroff J, Zapol WM, Picard MH. Echocardiographic determination of risk area size in a murine model of myocardial ischemia. Am J Physiol Heart Circ Physiol 277: H986–H992, 1999. doi: 10.1152/ajpheart.1999.277.3.H986. [DOI] [PubMed] [Google Scholar]
- 29.Senior R, Monaghan M, Main ML, Zamorano JL, Tiemann K, Agati L, Weissman NJ, Klein AL, Marwick TH, Ahmad M, DeMaria AN, Zabalgoitia M, Becher H, Kaul S, Udelson JE, Wackers FJ, Walovitch RC, Picard MH; RAMP-1 and RAMP-2 Investigators . Detection of coronary artery disease with perfusion stress echocardiography using a novel ultrasound imaging agent: two Phase 3 international trials in comparison with radionuclide perfusion imaging. Eur J Echocardiogr 10: 26–35, 2009. doi: 10.1093/ejechocard/jen321. [DOI] [PubMed] [Google Scholar]
- 30.Senior R, Moreo A, Gaibazzi N, Agati L, Tiemann K, Shivalkar B, von Bardeleben S, Galiuto L, Lardoux H, Trocino G, Carrió I, Le Guludec D, Sambuceti G, Becher H, Colonna P, Ten Cate F, Bramucci E, Cohen A, Bezante G, Aggeli C, Kasprzak JD. Comparison of sulfur hexafluoride microbubble (SonoVue)-enhanced myocardial contrast echocardiography with gated single-photon emission computed tomography for detection of significant coronary artery disease: a large European multicenter study. J Am Coll Cardiol 62: 1353–1361, 2013. doi: 10.1016/j.jacc.2013.04.082. [DOI] [PubMed] [Google Scholar]
- 31.Shimoni S, Frangogiannis NG, Aggeli CJ, Shan K, Quinones MA, Espada R, Letsou GV, Lawrie GM, Winters WL, Reardon MJ, Zoghbi WA. Microvascular structural correlates of myocardial contrast echocardiography in patients with coronary artery disease and left ventricular dysfunction: implications for the assessment of myocardial hibernation. Circulation 106: 950–956, 2002. doi: 10.1161/01.CIR.0000026395.19594.43. [DOI] [PubMed] [Google Scholar]
- 32.Sirsi SR, Flexman ML, Vlachos F, Huang J, Hernandez SL, Kim HK, Johung TB, Gander JW, Reichstein AR, Lampl BS, Wang A, Hielscher AH, Kandel JJ, Yamashiro DJ, Borden MA. Contrast ultrasound imaging for identification of early responder tumor models to anti-angiogenic therapy. Ultrasound Med Biol 38: 1019–1029, 2012. doi: 10.1016/j.ultrasmedbio.2012.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Villanueva FS, Glasheen WP, Sklenar J, Kaul S. Assessment of risk area during coronary occlusion and infarct size after reperfusion with myocardial contrast echocardiography using left and right atrial injections of contrast. Circulation 88: 596–604, 1993. doi: 10.1161/01.CIR.88.2.596. [DOI] [PubMed] [Google Scholar]
- 34.Wei K, Jayaweera AR, Firoozan S, Linka A, Skyba DM, Kaul S. Quantification of myocardial blood flow with ultrasound-induced destruction of microbubbles administered as a constant venous infusion. Circulation 97: 473–483, 1998. doi: 10.1161/01.CIR.97.5.473. [DOI] [PubMed] [Google Scholar]
- 35.Yuan LJ, Wang T, Kahn ML, Ferrari VA. High-resolution echocardiographic assessment of infarct size and cardiac function in mice with myocardial infarction. J Am Soc Echocardiogr 24: 219–226, 2011. doi: 10.1016/j.echo.2010.11.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.