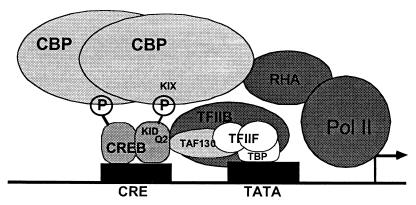
Figure 2.
The prevailing view for the mechanism of calcium-dependent CREB activation. CREB sits prebound as a dimer to the CRE in unstimulated cells. In response to neuronal stimulation and the activation of CREB kinases, CREB is phosphorylated at Ser-133 within the kinase-inducible domain (KID), allowing it to bind the KIX domain of CBP (or potentially a CBP dimer) recruiting this coactivator to the promoter. CBP promotes transcriptional activation in part through binding indirectly to the RNA polymerase II via an interaction with the RNA helicase A (RHA) protein, and thus CBP recruits the polymerase complex onto promoters bound by Ser-133-phosphorylated CREB. Other CREB-mediated interactions with the basal transcription machinery may also help to promote transcription. Through a glutamine-rich region (Q2), CREB binds to TAF130, a component of TFIID, and also to TFIIB. These complexes of proteins associate with the TATA binding protein (TBP) at the TATA box that is found just proximal to the initiation site of many genes. [Reproduced with permission from ref. 60 (Copyright 1999, Annual Reviews, http://AnnualReviews.org).]