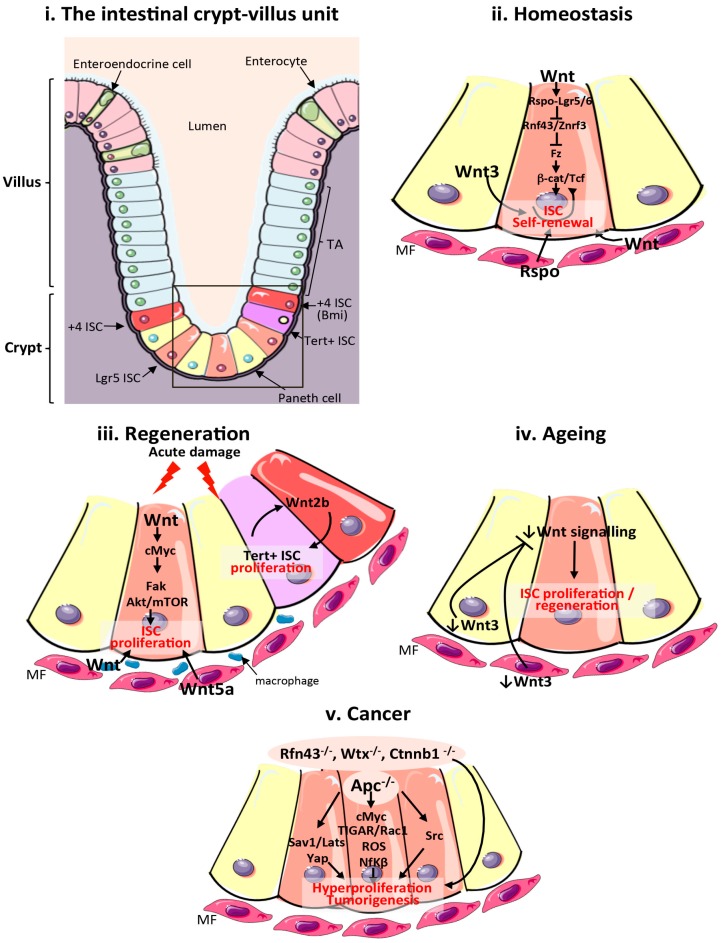
Figure 1.
Wnt signalling in the mammalian intestine during homeostasis, regeneration, ageing and cancer. (i) Schematic of the cellular composition and architecture of a crypt–villi unit in the mammalian intestine. The stroma is depicted in purple and the gut lumen in peach. The boxed area highlights the crypt and stem cell niche, which are magnified in (ii–v); (ii) Main sources of the Wnt stem cell niche and pathway activation during intestinal homeostasis. Wnt3 from Paneth cells, and Wnt and Rspo from mesenchymal and epithelial stem cell niches are important sources of Wnt during homeostasis; (iii) Sources of the Wnt stem cell niche and pathway activation during intestinal regeneration following damage. Wnt2b signalling from intestinal epithelial cells is required to activate proliferation of quiescent Tert + intestinal stem cells (ISCs). Wnt from macrophages and Wnt5a from the mesenchyme are also important sources of Wnt during regeneration. Wnt activation of cMyc is known to target Fak and Akt/mTOR pathways to increase ISC proliferation in response to damage; (iv) Reduced production of mesenchymal and Paneth cell Wnt3 and canonical Wnt pathway activity in the ageing intestinal epithelium impairs ISC proliferation; (v). Wnt pathway activation during intestinal tumourigenesis and functional pathways activated following Apc loss. ISCs: intestinal stem cells; TA: transit amplifying cells; Tert: telomerase reverse transcriptase; MF: mesenchymal fibroblasts; Apc: Adenomatous polyposis coli; Fak: focal adhesion kinase; ROS: reactive oxygen species; TIGAR: TP53-inducible glycolysis and apoptosis regulator; Rspo: R-Spondin; Sav1: Salvador 1; mTOR: mammalian target of rapamycin; Yap1: Yes associated protein 1; Lats: Large tumour suppressor kinase 1; Nf-κβ: Nuclear factor κβ.