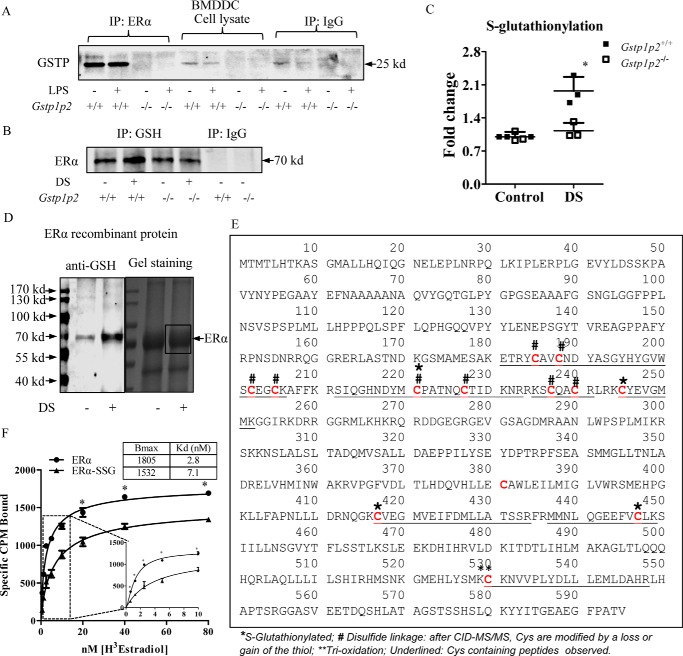
Figure 5.
GSTP catalyzes ERα S-glutathionylation through protein-protein interaction and regulates ERα binding affinity for estradiol. A, 500 μg of BMDDC lysates, untreated or treated with 200 ng/ml LPS (overnight), from WT and Gstp1/p2−/− mice were used for immunoprecipitation (IP) with anti-ERα antibodies. Samples were analyzed by SDS-PAGE with the IgG controls and blotted for GSTP. B and C, 500 μg of BMDDC lysates, untreated or treated with disulfiram (DS) to promote S-glutathionylation (10 μm for 30 min at 37 °C), from WT and Gstp1/p2−/− mice were used for immunoprecipitation with anti-GSH antibodies. Samples were analyzed by SDS-PAGE with the IgG controls, blotted for ERα, and quantified with Image Studio 4.0 software. Results are representative of three experiments. *, significant differences between Gstp1/p2−/− and WT, p < 0.05. D, recombinant ERα proteins, untreated and S-glutathionylated (10 μm disulfiram and 10 mm GSH for 30 min at 37 °C), were separated on a non-reducing gel. One part of the gel was transferred to a PVDF membrane and blotted with anti-GSH antibody (left panel), and the other part of the gel was stained by colloidal Coomassie stain (right panel). Bands (as indicated in the box) were excised, destained, enzymatically digested, and subjected to LC-MS/MS identification. E, proteomic identification of sites of cysteine modification on ERα (*, S-glutathionylated; #, disulfide linkage; **, trioxidation). F, saturation binding assays of estradiol. Recombinant ERα protein (untreated or S-glutathionylated) was incubated with [3H]estradiol (from 0.625 to 80 nm) at room temperature for 90 min, and bound estradiol was measured by liquid scintillation counting. Data were analyzed using a specific binding module from Prism 5.0. Maximum amounts of estradiol bound receptor (Bmax) and binding affinities (Kd) were calculated. Data shown are means ± S.D. of three experiments. Error bars represent S.D. *, significant differences between ERα and ERα-SSG, p < 0.05.