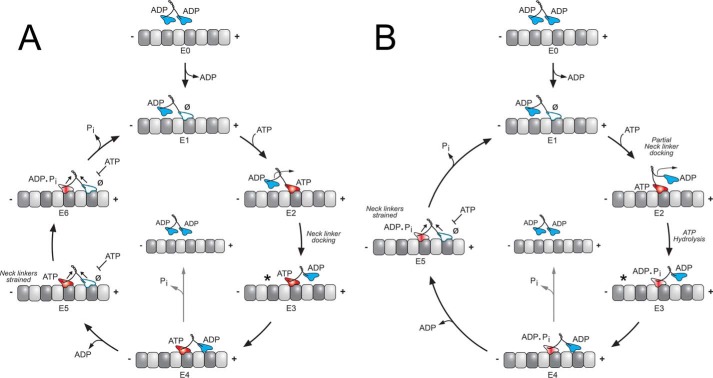
Figure 2.
KIF3 stepping models. Two variations of a kinesin stepping cycle are presented: a front-head–gated model (A), and a revised rear-head–gated model (B). Each cycle begins as one motor head collides with the microtubule; ADP is released, and the asymmetry of the ATPase cycle on each motor domain is established (E0–E1). The E1 intermediate is tightly bound to the microtubule with its leading head nucleotide-free and the trailing head detached as the weak-binding ADP state. A, ATP binds to the leading head and generates a structural transition transmitted through the neck-linker motif (E2–E3). The ATP-induced structural transition is designated neck-linker docking (*) and shifts the lagging unbound head forward by 16 nm to the next microtubule-binding site toward the microtubule plus-end (E2–E4). ADP is subsequently released (E4–E5). Both heads are bound to the microtubule with the leading head now nucleotide-free and tightly bound to the microtubule. ATP hydrolysis on the rear head (E5) results in another series of structural transitions in which phosphate is released; the trailing head transitions into a weakly bound ADP state and detaches from the microtubule to form the E1 intermediate. The first 8-nm step of the cycle is coupled to one ATP turnover and positions the new leading head to begin the second step of the processive run waiting for ATP (E1). The front-head–gated model (A) proposes that ATP binding promotes neck-linker docking that is coupled with a structural step (E2–E5) and that in the two-head bound state (E5), and ATP binding on the leading head is inhibited. In contrast, the revised rear-head–gated model (B) proposes that ATP binding partially docks the neck linker onto the catalytic core but posits that ATP hydrolysis (E2–E4) occurs while the tethered head is in its diffusional search for its microtubule-binding site with ATP hydrolysis required to completely dock the neck linker. The rear-head–gated model proposes that the E3–E4 intermediate is in a kinetic race for the front head to bind tightly to the microtubule before phosphate is released on the rear head (E4–E5).