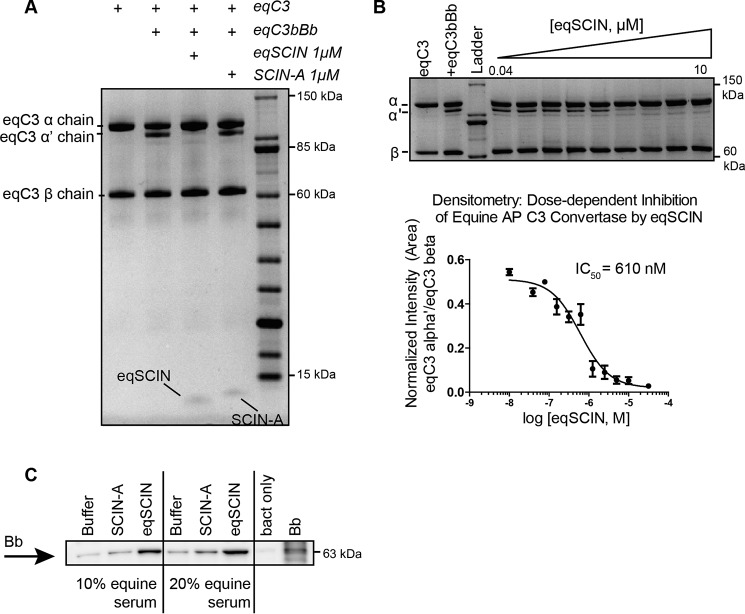
Figure 3.
eqSCIN interferes with equine C3 convertases. A, the ability of eqSCIN or SCIN-A to inhibit the fluid-phase equine AP convertase was assessed. An equimolar solution of eqC3b and equine factor B was mixed with human factor D to form an equine AP convertase (eqC3bBb). Equine C3 was mixed in the presence or absence of 1 μm eqSCIN or SCIN-A for 1 h at 37 °C. The conversion of eqC3 α-chain to α′ was monitored on a reducing SDS-polyacrylamide gel. In the presence of 1 μm eqSCIN, this conversion is inhibited, whereas in the presence of SCIN-A, the reaction proceeds in a manner similar to buffer control. B, a 2-fold serial dilution of eqSCIN (0.04–10 μm) was incubated with eqC3bBb, and the band corresponding to the eqC3 α′-chain was quantified by densitometry using ImageJ, where the invariant eqC3 β-chain was used to normalize each lane. These data indicate that whereas eqSCIN inhibits equine AP convertases, SCIN-A does not. All assays were performed in duplicate, and the half-maximal inhibitory concentration (IC50) was calculated using variable non-linear regression in GraphPad Prism version 5. C, eqSCIN stabilizes Bb on the surface of bacteria opsonized with 10 and 20% equine serum, and SCIN-A does not. Buffer only and 1 μm SCIN-A showed background levels of Bb, whereas Bb stabilization was increased by adding 1 μm eqSCIN. One representative experiment is shown of three independent experiments.