Figure 6.
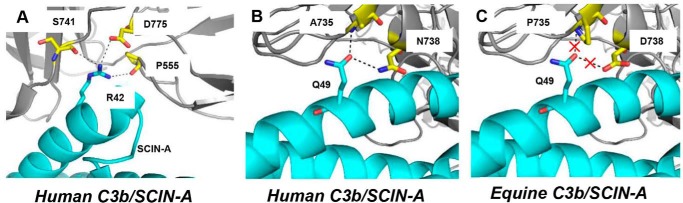
Modeling a putative SCIN-A/eqC3b interface. To gain insight into the human-specific nature of SCIN-A activity, the co-crystal structures of SCIN-A (cyan) in complex with human C3b (gray) were used to model an interaction between equine C3b and SCIN-A. A, SCIN-A Arg-42 forms hydrogen bonds with three human C3b residues (Pro-555, Ser-741, and Asp-775, marked in yellow) and is critical for mediating high-affinity SCIN-A/C3b interaction. An arginine residue is encoded at an equivalent position in eqSCIN, and C3b residues that directly contact Arg-42 are conserved between equine and human C3b. B, in contrast to the conserved Arg-42–mediated interaction, the Gln-49 residue of SCIN-A forms a salt bridge with human C3b residues Ala-735 and Asn-738 (shown in yellow), whereas in equine C3b (C), this interaction would be abrogated by A735P and N738D substitutions. The equivalent eqSCIN position encodes a Tyr residue rather than a Gln.