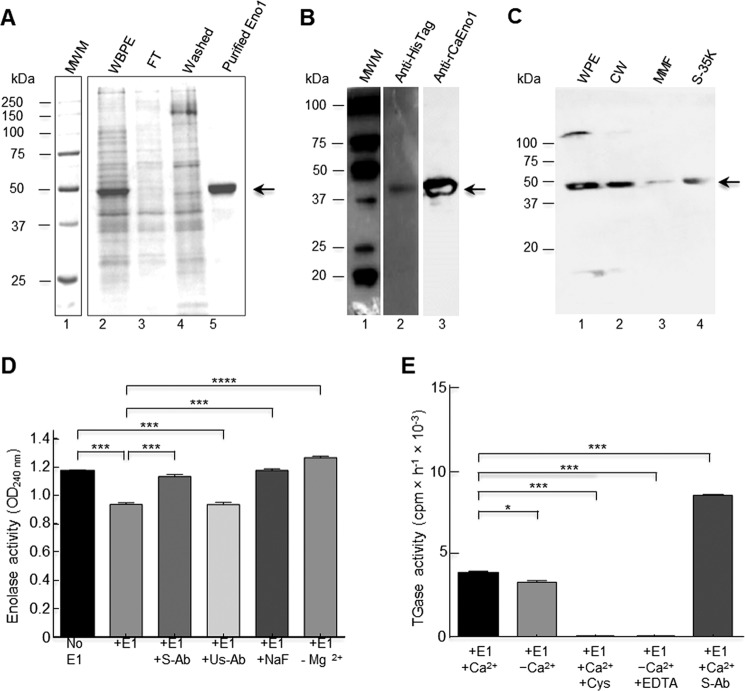
Figure 7.
Recombinant enolase1 from C. albicans has TGase activity. The C. albicans ENO1 gene was cloned in the pCold II plasmid and transformed into E. coli BL21 (DE3) pLysS-competent cells; protein production was induced at 23 °C for 24 h. A, Eno1 protein was purified by IMAC with a Ni2+-NTA–agarose column in native conditions as described, and elution fractions were evaluated by 12% SDS-PAGE; MWM, protein molecular weight markers. Empty vector was also transformed in bacteria and passed through the same IMAC column, and the fractions obtained were also analyzed as a negative control (data not shown). B, Western blot of purified recombinant protein using anti-His–tag polyclonal antibodies (lane 2) and rabbit anti-rCaEno1 protein (lane 3). C, Western blot of C. albicans cell fractions using anti-rCaEno1 polyclonal antibodies. WPE, whole-protein extracts; CW, cell wall fraction; MMF, mixed membrane fraction; S-35K, soluble cytosolic fraction. Arrows indicate Eno1 protein. D, enolase activity was determined with purified rCaEno1 protein. E, TGase activity determined with purified rCaEno1 protein. These results allowed us to conclude that rCaEno1 protein has both enolase and transglutaminase activities. Statistical unpaired t test. *, p < 0.05; ***, p < 0.001; ****, p < 0.0001. Bars are shown with standard error of mean. E1, enolase 1; S-Ab, specific antibodies (anti-rCaEno1); Us-Ab, unspecific antibodies (anti-rEhPCNA); Cys, cystamine.